(1)
Department of Neurosurgery, St Elisabeth-Tweesteden Hospital, Tilburg, The Netherlands
Early anatomists failed to see order amidst the apparent chaos of the brain’s convolutions. It was only in the second half of the nineteenth century that studies of the topographical pattern of gyri and sulci gradually gained weight and importance. Alexander Ecker (1816–1887) was among the first who mapped the convolutions and sought for anatomical order among individual brains (Fig. 5.1). In the Introduction of his book, On the Convolutions of the Human Brain (1869), he wrote:


Fig. 5.1
Schematic brain from Ecker’s On the Convolutions of the Human Brain (1873). Ecker says about his illustrations that they ‘should be considered less in the light of pictures than of maps by the aid of which the traveller will be in a position to better shape his course in the district which he is exploring’ (Figure taken from Ecker, 1873 [1])
For men were wont to regard the convolutions as a series of folds without order or arrangement, and draughtsmen represented them much as they would a dish full of macaroni. It was only by degrees that certain sulci and gyri came to be recognized as more constant than others; but as long as attention was confined only to the fully-developed human brain, real progress was not possible. Comparative Anatomy and the History of Development—those beacons of Human Anatomy—have been also the first to shed light upon this dark corner; for it was the labours of Huschke and, in particular, of Gratiolet, directed towards the brain of apes, that have established the conformity, in structural style, of the brain of apes with that of man, and have thereby for the first time paved the way towards a comprehension of the latter. [1]
Ecker and his contemporaries considered embryology (i.e. the study of the developing brain) and comparative anatomy (i.e. the study of the brains of different mammals and primates) indispensable:
to learn some day or other to recognise a law for the formation of the convolutions – that is to say, to learn to recognise and comprehend the formation of the convolutions as a necessary consequence of certain mechanical antecedents in the growth of the brain and the skull. [1]
5.1 Ecker, Leuret and Gratiolet: Order Out of Chaos
Ecker especially wrote his book for the physician who studied the brain, ‘so that he may be capable of registering with accuracy the all-important observations upon the pathological changes in the cortex of the cerebrum’ [1]. He included a short but systematic guideline for practical identification of the convolutions in the Appendix, which is still very useful today.1 Ecker advises his readers always to make a sketch of the portion of the cortical surface where the convolutions are difficult to identify or have an abnormal arrangement. He specifically refers to the diopter of Lucae for this purpose (Fig. 5.2) and to the—now famous—wax models of Ziegler which Ecker had helped to design.2


Fig. 5.2
Anthropologists sought for methods to standardize their observations. They generally accepted that drawing, rather than photography, was the most accurate means of representing skulls, because the expert could then control the representation. One of the most famous examples is the device invented by Lucae. The figure shows a small skull that is immobilized and an observer who uses a diopter in his left hand. This setup assures that his gaze always has a perpendicular perspective on the object. The object is drawn on a glass plate, and the ink on the glass is later transferred to paper. The result will be a geometric projection of the object (Figure taken from Zimmerman, 2001 [9])
Credit, for naming of the gyri and sulci, should probably go to Louis Pierre Gratiolet (1815–1865) and his teacher François Leuret (1797–1851). Their two-volume book (1839; 1857) was a unique source for several generations; it was the first large work to offer an organized description of the convolutions (Fig. 5.3) [3, 4]. The second volume was written solely by Gratiolet because of the illness and death of Leuret. Their book also paid homage to Rolando by coining the term fissure of Rolando [3]. Luigi Rolando (1773–1831) had expressed the new concept of the regularity of convolutions 10 years earlier (Fig. 5.4) [5]. Gratiolet also completed a monograph of his own, Mémoire sur les plis cérébraux de l’homme et des primatès (Cerebral folds of man and the primates) [6]. However, he was initially reluctant to assign functions to the structures he named:
In a general manner, I agree with M. Flourens that the intelligence is one, that the brain is one, that it acts above all as a whole; but this does not exclude the idea that certain faculties of the mind stand in special relation, although not exclusively, with certain cerebral regions.3
In 1861, Gratiolet and Broca engaged in a famous dispute in the Anthropological Society in Paris. Broca, at that time, had established himself as the head of French anthropology. He had been the founder of the Society (in 1859) and already enjoyed a good scientific reputation. Gratiolet’s work, in stark contrast, was greatly undervalued during his life and he lived in great poverty and hardship [7]. The topic of the debate was the relation between intelligence and brain size, a common topic of discussion at that time. Broca was a strong proponent of a direct and causal relationship and sought evidence for why some individuals or groups were more successful than others:
In general, the brain is larger in men than in women, in eminent men than in men of mediocre talent, in superior races than in inferior races. Other things equal, there is a remarkable relationship between the development of intelligence and the volume of the brain.4
It is here where anthropology, phrenology and evolutionary theories meet. ‘Races’ were compared and ranked according to scientific measurements, including those of the skull and the brain [9]. A good friend of Broca, Alphonse Bertillon (1853–1914), was a Parisian police officer who believed that criminals could be recognized on the basis of physical characteristics. He developed techniques and instruments to measure various features that would not change in adult life, e.g. eye colour, the shape of the ears and the distance between the eyes. One of the most famous proponents of ‘anthropometrics’ was Cesare Lombroso (1835–1909), an Italian physician and psychiatrist. His work on criminality, which is now discredited, laid the foundations for modern criminology. In his book Criminal Man, he argued that some people were born criminals and that they were throwbacks (atavistics) to a primitive stage of evolution. Lombroso believed that this primitiveness could be read from their bodies and their habits. His theories were met with fierce criticism, as others argued that criminals were not genetically predisposed but rather a product of social inequality and poverty. In later years Lombroso acknowledged that criminality is the result of both individual and social factors. Despite his views he was one of the first who advocated the rehabilitation and humane treatment of prisoners, in particular because he considered them not responsible for their own behaviour.
Broca greatly contributed to anthropology, not only because he devised numerous measuring devices but also because he systematically questioned the generally made assertions about ‘inferiority’ of races and thereby pointed out several fallacies [3]. But, as Schiller (1992) puts it in his biography of Broca:
We must not expect a Broca, a Lincoln, indeed any enlightened minds, to have believed in racial equality. The attitude was humanitarian, at best. Plain common sense, and even the most careful observation by the means then available, clearly showed that other races were unable to meet white standards measured by white values, in science, technical achievement, or art. [3]
Broca and Gratiolet share a common history. Coincidentally, they were both born in Sainte-Foy-la-Grande, a small village some 70 km east of Bordeaux.5 Both had also been students of Leuret. Their debate lasted for 5 months and centred around the massive head and large brain of Georges Cuvier (1769–1832), one of the scientific giants of that era. Cuvier’s brain weighted 1830 g, 400 above the average. Steven J Gould wrote a fascinating and amusing essay on the debate title ‘Wide hats and narrow minds’; here is an excerpt:
Thus, when Cuvier died, his colleagues, in the interest of science and curiosity, decided to open the great skull. (…) They began with the internal organs and, ‘finding nothing very remarkable’, switched their attention to Cuvier’s skull. ‘Thus’, wrote the physician in charge, ‘we were about to contemplate the instrument of his powerful intelligence.’ And their expectations were rewarded. (…) Broca pushed his advantage and rested a good part of his case on Cuvier’s brain. But Gratiolet probed and found a weak spot. In their awe and enthusiasm, Cuvier’s doctors had neglected to save either his brain or his skull at all. The figure of 1830 g for a brain could not be checked; perhaps it was simply wrong. Gratiolet sought an existing surrogate and had a flash of inspiration: ‘All brains are not weighted by doctors’, he stated, ‘but all heads are measured by hatters and I have managed to acquire, from this new source, information which, I dare to hope, will not appear to you as devoid of interest.’ In short, Gratiolet presented something almost bathetic in comparison with the great man’s brain: he had found Cuvier’s hat. And thus, for two meetings, some of France’s greatest minds pondered seriously the meaning of a worn bit of felt. [8]
5.2 Microscopic Cartography
Over the course of the nineteenth century, the microscope was improved to such an extent that it became possible to visualize individual brain cells and characterize their structure and architecture. Investigators such as Jan Purkinje (1787–1869), Otto Deiters (1834–1863), Wilhelm His (1831–1904) and Theodor Meynert (1833–1892) described different types of nerve cells with their axons and dendritic branches [10]. What was also observed was that these nerve cells formed ‘nets’ and were somehow connected to one another (Fig. 5.5). This posed the question whether the nervous system was one huge reticulum or made up of individually and anatomically distinct units. Were nerve cells directly connected to each other (as believed by the reticulists) or was there a gap between cells (as believed by the neuronists)? This ‘neuron doctrine’ was the topic of a long (and often bitter) scientific struggle, personified by two great names that both of whom received the Nobel Prize for their work in 1906: Camillo Golgi (1843–1926) and Santiago Ramón Cajal (1852–1934). The work of Cajal eventually provided most of the evidence for what Heinrich Waldeyer (1836–1921) would name ‘the neuron’. Cajal proved that axons communicate with other cells across a gap (later named synapse by Sherrington), although the synaptic space itself was only made visible with electron microscopy well into the twentieth century. For an in-depth and fascinating view, I recommend the book of Rapport, which tells the story of Golgi and Cajal from both a neuroscientific and a romantic historical perspective [11].


Fig. 5.5
von Kölliker’s 1867 portrayal of spinal nerve branches forming nets (Figure taken from Finger, 2001 [10])
The fact that neurons seemed independent units fueled early twentieth-century concepts that different brain areas carry specific functions and operate independently of each other. Even more so did the discovery that the cerebral cortex is made up of different cellular layers (Fig. 5.6) and the fact that there are large differences in layer architecture between different cerebral regions. Several anatomists each came up with their own cytoarchitectonic maps, the most famous ones being Brodmann, Campbell, Vogt and Vogt, Smith and von Economo and Koskinas (see Figs. 5.7 and 5.8) [12]. For some reason, it is predominantly the work of Korbinian Brodmann (1868–1918) that is remembered from this period. His maps of the cerebral cortex have been widely reproduced in the neurological and neuroscientific literature and almost become archetypical figures. ‘Brodmann areas’ (BA) are still frequently used in modern neuroscience to indicate brain areas and have in particular been closely linked, or even become synonymous with specific functions: for instance, BA 4 for primary motor functions, BA 17 for visual functions and BAs 44 and 45 as synonym for Broca’s area and ‘motor language’ functions. Despite all this, Brodmann’s famous book of 1909, entitled Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellensbaues, was not reprinted until 1985 and was only translated in English in 19946. The editor and translator, Garey, himself a neuroscientist, wrote in his Translator’s introduction:


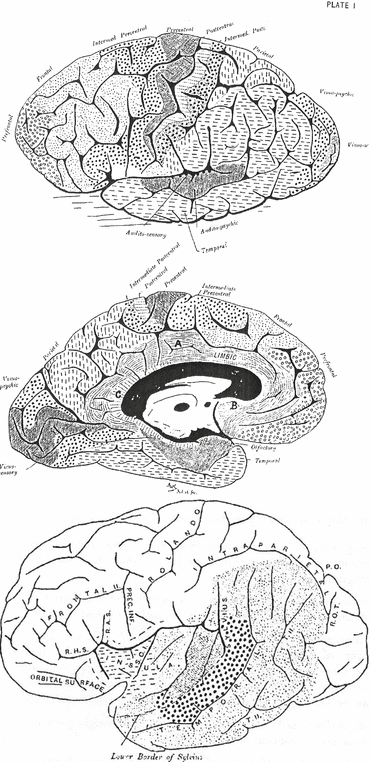

Fig. 5.6
Meynert’s 1885 representational drawing of the then known five numbered layers of the cerebral cortex (left), compared with Cajal’s detailed drawing of the same layer published 7 years later (right). Cajal later described the now accepted sixth layer (Text and figures taken from Rapport, 2005 [11], in which references are given to the original works of Meynert [69] and Cajal [70])

Fig. 5.7
(Top) Cytoarchitectonic maps of the lateral and medial surfaces of the human cerebral hemispheres. (Bottom) Insular region and superior aspect of the superior temporal region exposed. J. ant. agranular insular zone, J. post. granular posterior insular zone, sp posterior ramus of the Sylvian fissure, sv vertical ramus of the Sylvian fissure, sh horizontal ramus of the Sylvian fissure, t1 superior temporal sulcus. On the superior aspect of the superior temporal gyrus are three areas: 52 parainsular area, 41 anterior or medial transverse temporal area, and 42 posterior or lateral transverse temporal area (Figures taken from Brodmann’s Localisation in the Cerebral Cortex [13])

Fig. 5.8
Campbell’s schematic drawing of the human brain (1905). (Top) ‘Human brain, M. aet. 41. Orthogonal tracings of the lateral and mesial surfaces (the former somewhat tilted to show the convexity) of the left cerebral hemisphere, with a representation of the extent of the various areas defined therein from an examination of the cortical nerve fibres and nerve cells.’ ‘In a surface diagram it is impossible to give a true idea of many of these fields, because cortex concealed within fissures cannot be indicated, and unfortunately the figures are especially misleading in regard to some of the most important areas; thus the floor, not the lip, of the fissure of Rolando is the boundary between the precentral and postcentral fields, and accordingly the concealed portion of these areas is almost equivalent to that exposed: the same applies to the calcarine or visuo-sensory field, while that marked “audito-sensory” is almost completely hidden in the Sylvian fissure.’ (Bottom) ‘Drawing of the left cerebral hemisphere (human) with Sylvian fissure opened out. Showing (1) the audito-sensory area (shaded) confined to the two transverse temporal gyri and not extending on to the insula; (2) the audito-psychic area (large dots) on the free surface of the posterior three-fifths of the first temporal gyrus; (3) the extent, on the lateral surface of the hemisphere, of the common temporal cortex (small dots).’ SCI sulcus centralis insulae, RHS ramus horizontalis Sylvii, RAS ramus ascendens Sylvii, Prec. Inf. sulcus precentralis inferior, PO parieto-occipital fissure, ROT ramus occipitalis transversus, TH sulcus temporalis secundus (Text and figures taken from Campbell, 1905 [19])
Few people have ever seen a copy of the 1909 monograph, and even fewer have actually read it! There has never been an English translation available, and the original book has been almost unavailable for years, the few antiquarian copies still around commanding high prices. As I, too, used Brodmann’s findings and maps in my neurobiological work, and have the good fortune to have access to a copy of the book, I decided to read the complete text and soon discovered that this was much more than just a report of laboratory findings of a turn-of-the-century neurologist. It was an account of neurobiological thinking at that time, covering aspects of comparative neuroanatomy, neurophysiology, and neuropathology, as well as giving a fascinating insight into the complex relationships between European neurologists during the momentous times when the neuron theory was still new. [13]
5.2.1 Brodmann
Brodmann initially worked on the instigation of Oskar Vogt and Cécile Vogt-Mugnier in their neurological centre (Neurologische Zentralstation) in Berlin. Between 1901 and 1909 Brodmann systematically described the cytoarchitectonic architecture of the mammalian cortex. Brains were generously donated by the nearby zoological garden, whereafter they were fixed in formalin, embedded and sectioned in paraffin and then stained with cresyl violet. In this manner Brodmann meticulously analysed 63 different species, ranging from rat and cat to seal, tiger and chimpanzee. This was in addition to a number of human brains. It remains unclear on how many human specimens Brodmann’s work is based. The exact answer is not known (to me), but is certainly more than one, as Brodmann repeatedly mentions regional variations in microscopic and macroscopic (e.g. sulcal) borders between individuals. Brodmann and his contemporary cartographers all provide a comparative neuroanatomical approach because they believed in ‘the important biological principle that the genesis of mammalian cortex is not only conceived according to a common plan, but it completes its further development according to standard rules’ [13]. As Zilles and Amunts (2010) write in their ‘Centenary of Brodmann’s map’:
Based on this integrative concept (histology with phylogeny), Brodmann indicated through his numbering system homologies between the cortical areas of different mammals. (…) Implicitly, Brodmann demonstrated that the architectonic parcellation of the human cortex can be understood only by comparison with different mammalian brains. (…) Each cortical area of his human map is labelled by a number between 1 and 52, but areas with the numbers 12–16 and 48–51 are not shown in his map. Brodmann explained these ‘gaps’ with the fact that some areas are not identifiable in the human cortex but are well developed in other mammalian species. This holds true particularly for the olfactory, limbic and insular cortices. [12]
Brodmann thus divided the human cerebral cortex into 43 areas. According to Zilles and Amunts:
Only those regional differentiations in the cortical structure had been taken into account, which are apparent in the laminar organization of a cross-sectioned gyrus, in the positioning, size, packing density and distribution of cells, that is, in the cytoarchitectonic differences. Histological differences sensu strictu, that is, details of single cells, appearance of fibrils and tigroid substance as well as details of the structure of the cell nucleus, etc., are not used topographically. [12]
It is important to note that Brodmann also did not include white matter architecture. ‘Myeloarchitecture’ was studied by the Vogts who used myelin-stained histological sections for this purpose. With their findings they further subdivided the 43 areas of Brodmann into approximately 200 areas, adhering to the major cortical areas as described by Brodmann. Although Brodmann areas are today still frequently used in scientific papers, they do not account for regional differences in fibre connectivity, a fact that is not often realized. This is important because modern neurocognitive theories focus more and more on the dynamic interaction between areas and not only on their localized functions. Brodmann was convinced that cytoarchitectonically different areas must subserve different functions. However, he did not conclude from this that functions were necessarily located in only one area. He seemed well aware of the limitations of his work and in particular of the ‘functional’ conclusions that could be drawn from it:
I remain hopeful that the results of histological localisation will not be without influence on the histopathology of the cerebral cortex. I am however not so optimistic as to believe that areal topography, as I have described in this treatise, will at present lead to cortical localization of individual psychiatric disorders or even individual psychological symptoms. [13]
Still, Brodmann discusses the possible functional consequences of his work in the last chapter of his book, Physiology of the cortex as an organ:
Although my studies of localisation are based on purely anatomical considerations and were initially conceived to resolve only anatomical problems, from the outset my ultimate goal was the advancement of a theory of function and its pathological deviations. Now the question arises as to what we can deduce from our histotopographical findings in terms of physiology of the cerebral cortex. [13]
Brodmann also reviewed contemporary opinions and quoted Meynert, Exner and Wundt that all considered nerve cells to play a subordinate role in functional differentiation. According to them, functional specialization mainly resulted from differences in excitation patterns between brain areas and not from differences within the grey matter itself. One of the great merits of Brodmann’s work is that he tried to refute localist concepts on histological and ontological grounds. In later work he added the results from lesion and stimulation studies in animals to support his views [14]. In proposing new hypotheses, Brodmann firmly disagreed with theories that reduce functional ‘concepts’ to specific cells, cellular layers or even brain areas:
The first thing to say is that just as untenable as the idea of a ‘concept cell’ or an ‘association layer’ is the assumption of specific ‘higher order psychic centres’. Indeed recently theories have abounded which, like phrenology, attempt to localize complex mental activity such as memory, will, fantasy, intelligence or spatial qualities such as appreciation of shape and position to circumscribed cortical zones. Older authors such as Goltz, Rieger, Wundt, and recently, particularly outspokenly, Semon, have already quite rightly expressed their opposition to such a ‘naive view’ and pleaded simple psychological facts against it. [13]
It was Brodmann’s strong opinion that the higher mental faculties, in particular, can only take place:
through an infinitely complex and involved interaction and cooperation of numerous elementary activities, with the simultaneous functioning of just as many cortical zones, and probably of the whole cortex, and perhaps also including even subcortical centers. Thus we are dealing with a psychological process extending widely over the whole cortical surface and not a localised function within a specific region. (…) One must therefore also assume a certain regional preference for higher activities, sometimes more in occipital and temporal areas, sometimes more in frontal. Such activities are, however, always the result (and not merely the sum) of the function of a large number of suborgans distributed more or less widely over the cortical surface; they can never be the product of a morphologically or physiologically independent ‘centre’. [13]
Brodmann thus did not pursue a strict localist view on function, and it was clear to him that language functions could not be linked to single brain areas.
It would be particularly tempting (…), considering the controversy recently engaged by Pierre Marie about aphasia [see chapter 1 of this book], to also engage in a discussion of the specific localisation of speech. However, it seems to us that the time is hardly ripe for this for most of the necessary physiological preparatory work is lacking. What is more, it is in no way to be seen as definite that the cortical localisation of speech coincides with that of aphasia. In relation to aphasia, however, one can already immediately conclude two things from the psychophysiological considerations described above. First, an aphasia, regardless of whether it belongs to the motor or sensory subcategory, can never be linked to a single structural centre, and always includes a complex of such areas, forming a larger region. Secondly, the ‘aphasia centre’ covers a much greater expanse than one was formerly accustomed to believe. [13]
Still, Brodmann mentioned ‘Broca’s area’ when he gave anatomical descriptions of areas 44 and 45. However, he did not do it very consistently: first he linked it to area 44, later to both areas. What is of more importance is that Brodmann identified the pars opercularis as the seat of area 44 and the pars triangularis as the seat of area 45. This connection of microscopically and macroscopically defined brain areas has become common practice and is still frequently taken for granted in neurocognitive studies. It is not justified, however, as there is no invariant relationship between cytoarchitectonic areas and gyral/sulcal topography, a fact that has nowadays been convincingly demonstrated [12, 14]. Macro-anatomical landmarks are not reliable in identifying cytoarchitectonic regions [15]. Brodmann was already well aware that there was no perfect match. In his descriptions of cytoarchitectonic areas, he explicitly mentions that these are approximately bounded by the various sulci. He also repeatedly commented on the intersubject variability of gyri and sulci, to the extent that he almost seemed to warn his readers. Here is his literal account of the areas of the inferior frontal gyrus:
Area 44—the opercular area—is a well-differentiated and sharply circumscribed structural region that on the whole corresponds quite well to the opercular part of the inferior frontal gyrus—Broca’s area. Its boundaries are, posteriorly, approximately the inferior precentral gyrus, superiorly the inferior frontal sulcus and anteriorly the ascending ramus of the Sylvian fissure. Inferiorly or medially it encroaches on the frontal operculum and borders on the insular cortex. The area then stretches around the diagonal sulcus, and there are again minor structural differences between the cortex in front of and behind this sulcus to justify the separation of an anterior opercular area from a posterior opercular area by the diagonal sulcus. As there is much variability and inconsistency of these sulci one will find rather mixed topographical relationships of these structural areas in individual cases.
Area 45—the triangular area—is cytoarchitectonically closely related to the previous area [area 44] that corresponds approximately to the triangular part of the inferior frontal gyrus. Consequently its caudal border lies in the ascending ramus of the Sylvian fissure, its dorsal border in the inferior frontal sulcus and its rostral border near the radiate sulcus of Eberstaller, although it may extend in places beyond this last sulcus as forward as the frontomarginal sulcus of Wernicke, and this area may also encroach partially on the orbital part; on the inferior surface of the inferior frontal gyrus it borders the insular cortex.
Concerning the exact morphological borders of the last two areas [44 and 45], that are so extremely important on account of their relationship to the motor speech area, I should like once again to expressly point out the great individual variations of the sulci in this region. As emerges from Rezius’ great monograph ‘Das Menschenhirn’, the diagonal sulcus is not infrequently fused with the inferior precentral sulcus or communicates with the ascending ramus, is often very strongly developed, but sometimes is entirely absent. The radiate sulcus and the ascending ramus vary widely in shape and structure so that naturally the relations of areas 44 and 45 to these sulci must be subject to major individual variations.(…)
Area 47—the orbital area—shares certain architectonic affinities with areas 44 and 45 such that it can be combined with them to form a subfrontal region. It lies essentially around the posterior branches of the orbital sulcus, generally well differentiated from area 11, but with constant morphological borders. Laterally it crosses the orbital part of the inferior frontal gyrus. [13]
Note that Brodmann thus grouped together areas 44, 45 and 47 on the basis of cytoarchitectonic similarities and clustered them into a subfrontal region. He assumes that this region is a more suitable candidate for Broca’s area than area 44 alone, although he does not provide any ‘functional’ evidence in his book. He also refrains from speculation about brain areas that might be related to ‘sensory aphasia’:
I have already made brief reference elsewhere to the fact that in particular, according to all that can be concluded from anatomical localisational data, the seat of motor aphasia must extend much further anteriorly than appears from Broca’s classic theory, and that at least the anterior sections of the inferior frontal gyrus, and perhaps even part of the actual orbital surface, must be included in it (thus, apart from area 44, also areas 45 and 47). [13]
A major drawback of Brodmann’s work is that his observed intersubject variability was not reflected in his maps. This simplification has undoubtedly led to misinterpretation of his work and further supported the dogma of a strict correspondence of brain structure and function. Along a similar line of reasoning, it can be said that Wernicke’s language schemes have been interpreted too literally; his idea that a large part of the temporal lobe was involved in language processing was generally overlooked in the historiography, presumably because Wernicke indicated language areas in his schemes only with small circles. The main reason for all this could well be that the maps and schemes of Brodmann and Wernicke are so visually appealing and convincing in their simplicity (as maybe any good model should be) that the textual nuances that were made by their authors got lost over time. It is also not easy to visualize uncertainty and variability across different individuals in a single schematic drawing. Nowadays, imaging studies use standardized and averaged brains for display of group results (see for an example Fig. 5.9). The disadvantage of these computerized brains is easily seen: details are lost and the cortical surface has a ‘smoothed’ appearance because intersubject variability has been largely averaged out. The brain is in fact—again—reduced to a fairly simple drawing, with only the ventricles and major sulci still identifiable. Statistical MRI atlases were developed to overcome the idiosyncrasies of using the brain of a single subject as a template. A more recent example of such a single brain atlas is the stereotactic atlas of Talairach and Tournoux (1988) (Fig. 5.10) [16]. For many years this coordinate system served as a guiding tool for neurosurgical procedures and also as the standard for reporting imaging results. Brodmann areas were added to later electronic versions of the atlas [17]. However, the original atlas was based on the post-mortem section of a single subject: a 60-year-old French woman with a smaller than average brain size. Most brains would need a significant deformation if they were to match such a ‘standard’ brain.



Fig. 5.9
MRI scans of the brains of 150 normal subjects were co-registered and transformed into a common stereotactic space, creating an ‘average’ brain. Note that details near the cortex are more blurred than deeper structures (e.g. basal ganglia) due to higher interindividual variability of cortical than subcortical structures

Fig. 5.10
Original digitized axial plate from the Talairach and Tournoux atlas (1988) (Figure taken from Nowinski and Belov, 2003 [71])
So which map is best? Or, as put by Jones in a critical review in Brain (2008):
Who can say whether the map of the human cerebral cortex by von Economo and Koskinas with its 107 areas is any more ‘correct’ than that of Campbell with 14, of Brodmann with 44, of the Vogts with more than 200 (…). [18]
Indeed, who can say? (By the way, note that Jones refers here to incorrect numbers of areas from the historical authors.) It may be apt to assume that the work of most authors has been largely forgotten because their maps have simply been surpassed in quality by those of Brodmann. However, it is not simply a question of ‘right’ and ‘wrong’. We have already seen the problems with any composite map due to intersubject variability. Another difficulty is that the historical maps were all the result of subjective visual inspection of histological sections. Investigators searched for more or less abrupt microscopic changes (for instance, in the presence of large pyramidal cells or the distinctiveness of the laminar and columnar organization of the cortex) in order to assess whether or not one area significantly differed from another [14]. This basically requires multivariate analysis and is better done with a computer (see Fig. 5.11). And, of course, maps are nearly impossible to compare because they are all influenced by the various presumptions and axioms that were made by their creators. Campbell’s maps, for instance, were not only based on findings from cytoarchitecture but also on myeloarchitecture and clinico-pathological deficits. It is therefore not surprising that the number and territories of his areas substantially differ from those of Brodmann.












