Natural Evolution and Resolution of Chronic Low Back Pain
Michel Benoist
Philippe Boulu
Pierre Guigui
Low back pain (LBP) is a frequent problem in adults from western societies. Cross-sectional surveys indicate a point prevalence of approximately 20% (1). The prognosis of acute LBP episodes is usually excellent, but a small proportion of sufferers experience persistent or relapsing episodes. According to insurance and industrial data, approximately 10% of sick-listed patients are still off work after 6 months (13). Epidemiology of chronic patients without compensation or work disability is less well known. This small proportion of chronic patients is responsible for most of the compensation and medical expenses linked to LBP.
To modify this unfavorable evolution, prevention measures have been developed, including exercise programs, ergonomic education, weight loss, and cessation of smoking. However, despite appropriate prevention, the prognosis remains less favorable in a subset of patients who develop a chronic problem, with intermittent exacerbations well beyond the expected period of healing, often extending months or even years after the initial visit.
When dealing with this category of patients, the treating physician must determine the cause of the pain and disability and subsequently define an appropriate therapeutic program. The purpose of this chapter is to discuss the neurophysiologic mechanisms involved in the chronicity of LBP and accordingly have an overview of the therapeutic strategies capable of hastening resolution of pain and disability in this category of chronic, often sick-listed patients.
Neurophysiologic Mechanisms of Chronic Low Back Pain
It is now admitted that pain transmission involves four main levels: the nociceptive input coming from peripheral tissues, the cord, the brain, and the descending inhibitory pathways (Fig. 4.1).
Peripheral Sensitization
Peripheral sensitization is defined by reduction of the nociceptive response threshold. In the case of LBP, the first level of the pain system is located in the spinal unit. Discogenic origin is generally assumed to be the major cause of nonspecific LBP. However, sources of nociception are also found in other structures of the motion segment including facets, ligaments, and muscles. Yet, it is generally admitted that degenerative changes in the
facets and ligaments follow degeneration of the intervertebral disc (4). In the case of pure LBP without radiculopathy, which is the topic of this chapter, the nerve fibers responsible for transmitting the pain message are located in the degenerated disc. Studies have shown the presence of nociceptive nerve fibers in the annulus and also in the inner nucleus of the intervertebral disc (5,19). The presence of innervation expressing substance P in the inner part of the annulus and in the nucleus has been demonstrated in the study by Freemont et al. (8) in degenerate discs painful at discography. Taken together these observations strongly suggest a role for these nerve terminals in the pathogenesis of LBP. An innervated disc can be a source of nociception. The nerve terminals can be sensitized in three different ways. Figure 4.2 presents a hypothetical and rough sketch of the biologic events leading to the sensitization of nerve terminals and the generation of LBP. First, neuropeptides, such as substance P and calcitonin
gene-related peptide, expressed by nerve fibers directly excite the nociceptors and induce a neurogenic inflammation by releasing inflammatory substances. Second, proinflammatory mediators, including prostaglandin E2 (PGE2) and cytokines [interleukin (IL)-6, IL-7, and IL-8] have been found at high levels in tissue cultures of patients undergoing lumbar interbody fusion for discogenic LBP (3). IL-1 and tumor necrosis factor (TNF) alpha have been detected in homogenates of contained subligamentous disc herniations inside the intervertebral space (22). These cytokines are potent stimulators of prostaglandins, which in turn sensitize the afferent pain fibers. In addition, neurotoxic properties have been demonstrated in the various cytokines present in the disc, particularly TNF alpha (21).
facets and ligaments follow degeneration of the intervertebral disc (4). In the case of pure LBP without radiculopathy, which is the topic of this chapter, the nerve fibers responsible for transmitting the pain message are located in the degenerated disc. Studies have shown the presence of nociceptive nerve fibers in the annulus and also in the inner nucleus of the intervertebral disc (5,19). The presence of innervation expressing substance P in the inner part of the annulus and in the nucleus has been demonstrated in the study by Freemont et al. (8) in degenerate discs painful at discography. Taken together these observations strongly suggest a role for these nerve terminals in the pathogenesis of LBP. An innervated disc can be a source of nociception. The nerve terminals can be sensitized in three different ways. Figure 4.2 presents a hypothetical and rough sketch of the biologic events leading to the sensitization of nerve terminals and the generation of LBP. First, neuropeptides, such as substance P and calcitonin
gene-related peptide, expressed by nerve fibers directly excite the nociceptors and induce a neurogenic inflammation by releasing inflammatory substances. Second, proinflammatory mediators, including prostaglandin E2 (PGE2) and cytokines [interleukin (IL)-6, IL-7, and IL-8] have been found at high levels in tissue cultures of patients undergoing lumbar interbody fusion for discogenic LBP (3). IL-1 and tumor necrosis factor (TNF) alpha have been detected in homogenates of contained subligamentous disc herniations inside the intervertebral space (22). These cytokines are potent stimulators of prostaglandins, which in turn sensitize the afferent pain fibers. In addition, neurotoxic properties have been demonstrated in the various cytokines present in the disc, particularly TNF alpha (21).
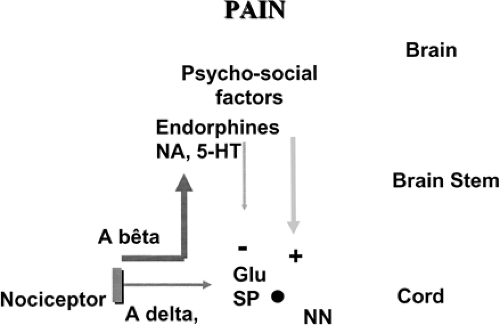 FIGURE 4.1 Pain is a complex phenomenon involving not only peripheral nociception but also the central nervous system. |
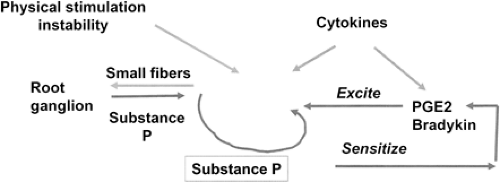 FIGURE 4.2 Hypothetical sketch of peripheral sensitization of nerve terminal found in degenerate discs. |
Third, disc degeneration can contribute to the production of nonspecific LBP through mechanical mechanisms. Tears and clefts of the disc and a diminution of disc height, which are the macroscopic hallmarks of disc degeneration, lead to mechanical instability of the motion segment. Even micromovements may cause pain when sensitized nerve terminals are stimulated.
Central Sensitization in the Spinal Cord
Central sensitization is defined by an increase of the nociceptive spinal neurons’ excitability. There is now evidence that sensitization of the central nervous system (CNS) can enable persistent pain states. Animal models of chronic pain have demonstrated that the spinal cord is a major contributor in the maintenance of chronic pain after peripheral nerve damage (6). Long-lasting stimulation of the neurons of the dorsal horn of the cord by neurotransmitters (substance P, glutamate) and neuromodulators induces plasticity and reorganization of the whole dorsal horn synapse. Studies have also shown that activated glial cells produce proinflammatory cytokines inducing in turn algesic mediators enhancing the nociceptive activity. Neuroimmune activation and neuroinflammation of the cord following nerve injury have been demonstrated in a rodent model (29). This permits development and amplification of painful perception independent of a primary afferent drive. It could explain the mismatch between chronic LBP and the poor physical and imaging findings.
The Brain
There is also increasing evidence that in addition to the spinal cord the higher cortical centers play a role in CNS central sensitization and the perception of pain. A functional imaging study using functional magnetic resonance imaging (MRI) has compared a group of chronic LBP patients without major psychosocial factors or any anatomic abnormalities on plain radiographs or spinal MRI that could explain the symptoms with a group of healthy controls (11). Experimental pain testing was performed at a neutral site by increased pressures on the thumbnail to assess the pressure pain threshold. The pressure required to produce intense pain was significantly higher in the controls, demonstrating hyperalgesia in the chronic LBP group. At pressure pain threshold, an increase in cerebral blood flow was observed in multiple pain-related regions of the brain compared with a single activation in controls. Interestingly, when stimuli generated equally painful responses in the two groups, the neuronal activated areas were similar. These findings indicate an augmentation of central nociceptive activity in a certain group of chronic LBP patients in whom all the known anatomic or psychosocial
factors did not explain the symptoms. The same investigation was applied to a group of fibromyalgia patients who experienced hyperalgia and altered brain processing similar to the chronic LBP group. The cortical nociceptive hyperactivity may also excite the nociceptive spinal neurons by putative glutamatergic descending excitatory pathways originating from the activated brain areas.
factors did not explain the symptoms. The same investigation was applied to a group of fibromyalgia patients who experienced hyperalgia and altered brain processing similar to the chronic LBP group. The cortical nociceptive hyperactivity may also excite the nociceptive spinal neurons by putative glutamatergic descending excitatory pathways originating from the activated brain areas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








