Introduction
Simple sensory seizures represent challenging clinical phenomena. Neurologists readily accept that a patient’s sensory symptoms prior to the appearance of motor symptoms or alteration of consciousness represent part of the patient’s clinical seizure semiology and provide valuable localizing information. A challenge appears when a patient has only simple partial sensory seizures. Simple partial seizures are a relatively common seizure presentation among the childhood epilepsies, but motor seizures are predominant.47 The paucity of psychic or sensory symptoms may reflect the child’s inability to describe internal experiences in a way that is easily interpretable to adult observers. Since simple sensory seizures have no overt ictal behavior, the subjective description of the seizure symptomatology is the only clue that can lead to a proper clinical evaluation and treatment. Some patients react to sensory seizures in a stereotypic fashion. For example, a painful simple partial sensory seizure of the hand may result in hand rubbing or vocalization about the pain. Occasionally, physicians encounter patients who have simple partial seizures for many years prior to the appearance of more clinically apparent seizures. When patients with pure sensory seizures also have psychiatric complaints, the chances for misdiagnosis or delay of diagnosis are high.121
Simple sensory seizures were originally underrecognized as epilepsy.35 While auras were known in ancient times as an event preceding seizures,122 it was not until the early 19th century that partial seizures were clearly related to localized brain disease. Fritsch and Hitzig described localization of function in 1870, which led to modern concepts of cortical specification.38
Jackson also promoted the hypothesis that abnormal electrical activity in a localized region of cortex could cause symptoms such as those seen in epilepsy.51,52 In the early 1900s, the work by Brodmann18 and other neuroanatomists showed that the cerebral cortex had regional histologic variations often corresponding to different cerebral functions. Penfield, Jasper, and others made major contributions to mapping the localization of cerebral function and defining motor and sensory areas of the brain, as well as areas without obvious effect on sensory, motor, or speech activity.83,85
Definitions
Sensory seizures were defined by Penfield and Jasper separately from simple seizures with autonomic symptoms or psychic symptoms:85
“Sensory seizures. Discharge in an area of sensory representation produces a sensation that may serve as a warning (aura) of a motor or psychical seizure. Actually, the sensation constitutes, in itself, a seizure. Under this heading we have taken up the sensory attacks that involve the trunk, head, and extremities (somatosensory), and the special senses (visual, auditory, vertiginous, gustatory, and olfactory).
Somatosensory seizures. Sensation of this type may be transient, or it may be continuous over a considerable period of time. The quality of sensation is tingling, numbness, sense of movement, desire to move, or, very occasionally, pain. A detailed march of sensation may occur from one somatic part to the next in a manner similar to the Jacksonian motor march. The discharge, which produces this, usually occurs in the postcentral gyrus. There is a tendency for the sensory march to stop and to spread across the Rolandic fissure so as to produce movement in the same member. Spread in the reverse direction from motor to sensory representation, thus producing sensation after movement, is quite rare.
A somatosensory seizure may also be produced by discharge in the second sensory area on the upper bank of the fissure of Sylvius. Furthermore, peculiar body sensations may result from discharge in the supplementary motor area within midsagittal fissure.
Visual seizures result from discharge in one or the other occipital lobe. Lights, which may be colored, appear before the patient, or there may be dimming of vision and complete blindness even though the discharge originates in one occipital lobe. Complicated visual hallucinations and alterations in the interpretation of things seen are not included in this group, but are discussed under the heading of psychical seizures.
Auditory seizures are characterized by a simple sound, usually described as buzzing or drumming, and often referred by the patient to the opposite ear, or opposite side of the head. Like a visual seizure, there is nothing elaborate about it. Hallucinations of music, for example, are classified as dreamy states or psychical seizures and are discussed below.
Vertiginous seizures. What is called by the patient dizziness or unsteadiness is frequently reported, but there is rarely any recollection of direction of rotation as there is in the sudden dizziness of Meniere syndrome.
Olfactory seizures have been called uncinate fits because of the demonstration by Jackson of involvement of the uncinate gyrus in cases in which an aura of disagreeable odor constituted the initial feature. The localization of discharge is doubtless in or near the uncinate gyrus.”
Penfield and Jasper’s classification of simple partial sensory seizures has withstood the test of time since the international classification26 of simple partial seizures with somatosensory or special sensory symptoms is nearly identical to the original description except for lack of description of gustatory seizures (Table 1). It is unlikely that another sensory modality will be discovered and change the classification further.
Table 1 Simple Partial Seizures with Somatosensory or Special Sensory Symptomsa | ||
|---|---|---|
|
Penfield and Jasper recognized the difficulty of separating a simple sensation from a more complicated hallucination, for example, separating simple visual hallucinations from more complicated formed illusions. Occasionally one finds more complicated hallucinations with intraoperative stimulation, but more reliably localizing are the simple hallucinations of rather elementary sensory perceptions. A conscious patient may attach many complex descriptions to simple
hallucinations, and since we observers have nothing to observe other than the patient’s subjective description, sometimes there may be a fine line between a classification of simple partial sensory seizure and a simple partial psychic seizure with prominent sensory complaints.
hallucinations, and since we observers have nothing to observe other than the patient’s subjective description, sometimes there may be a fine line between a classification of simple partial sensory seizure and a simple partial psychic seizure with prominent sensory complaints.
Epidemiology
Ideally, understanding the epidemiology of simple partial seizures would require evaluation of a large population in which patients with epilepsy are identified accurately and their seizures and epilepsies are classified based on a thorough clinical and laboratory investigation. Such studies are usually based on patients referred to specialized epilepsy centers. Focal epilepsy occurs in approximately 60% of patients with epilepsy, and 10% to 21% of these patients have simple partial seizures alone.40 Detection of simple partial seizures can be difficult. Some patients with simple partial sensory seizures may be undiagnosed or unaware that their symptom represents epilepsy unless the seizure progresses into a more disabling seizure type. In patients with obvious epilepsy and more severe types of seizures, only a careful history will elicit remote sensory complaints that may no longer occur, as in several cases reported by Williamson et al.123
Isolated simple partial sensory seizures are uncommon in patients referred to major epilepsy centers. Among the focal epilepsies, temporal lobe and frontal lobe foci predominate in surgical series.119 Parietal and occipital cases are rare because those epilepsies are either rare, rarely intractable, or rarely brought to surgery. For example, occipital epilepsy was identified in only 12 of 502 nontumoral epilepsy cases by Bidzinski et al.12 Williamson et al. reviewed reports of occipital lobe epilepsy in the literature and found that <2% of focal epilepsy is occipital.124 They found only sporadic reports when they reviewed parietal lobe epilepsy.123
These surgical data are typically grouped by lobe, not seizure type. It is likely that the epidemiology of simple partial sensory seizures differs from the lobar distribution of surgical cases. A review of the initial signs of seizure onset in data adapted from Penfield and Kristiansen showed that in 222 patients, 55 had somatosensory auras, 11 had visual auras, three had auditory auras, one had an olfactory aura, and two had gustatory auras.62 Penfield and Perot noted visual hallucinations in 41 of 1,132 (3.6%) patients with focal epilepsy; 21 had only visual hallucinations.87 Mauguiere and Courjon found somatosensory epilepsy in 127 of 8,938 (1.4%) patients with epilepsy when evaluated after age 3 years.72 Young and Blume reported painful seizures in 24 of 858 (3%) epileptic patients129 and Hausser-Hauw and Bancaud noted that 30 of 718 (4%) intractable epileptics had gustatory hallucinations.43 West and Doty reviewed olfactory auras in epilepsy and concluded that prevalence is unknown but published estimates ranged from <1% to >30% depending on the epilepsy syndrome and pathology.122
The advent of magnetic resonance imaging (MRI) technology has further complicated matters. Patients with lesions identified in known sensory cortical regions may not volunteer information on sensory symptoms until thoroughly interrogated about an aura. At times, only with inpatient video-electroencephalography (V-EEG) and MRI can sensory symptoms be suspected and assessed. This is especially true in patients with sensory symptomatology at seizure onset that progresses to loss of consciousness and amnesia for the aura. These patients often have more intense seizures and EEG changes are usually bilateral.103 Additionally, sensory symptoms may result from spread of a seizure from an unexpected location; for example, many patients with supplementary motor seizures complain of a somatosensory aura even when lesions are not near the sensory cortex.106 Gustatory hallucinations may also represent seizure spread.43 Visual hallucinations have been reported with frontal lesions,102 as have jacksonian sensory seizures from a prefrontal lesion.118 Thus, at this time, there is still need for adequate epidemiologic studies of localization-related epilepsies and partial sensory seizures using modern technology and appropriate clinical information.
Anatomic Pathways and Pathophysiology
Understanding neocortical anatomy and regional interconnections has led to a better understanding of seizure semiology and evolution. The neocortex is a repository for all primary sensory input for somatosensory, visual, auditory, and gustatory senses, while olfaction mostly utilizes portions of the limbic system and is the only special sense without thalamic relays.21 Within the cerebral cortex, sensory functions are not so discreetly organized that there are sharp boundaries; for example, the sensory responses obtained by Penfield and Rasmussen88 and Uematsu et al.116,117 showed that sensory responses could be recorded from the precentral gyrus (see reference 85, p. 58, Fig III-12). Motor responses show similar dispersion. Penfield reported that removal of the precentral gyrus did not prevent motor responses from the postcentral gyrus, suggesting that activation of the postcentral gyrus alone could result in movements during seizures.
The sensory homunculus (see reference 85, p. 70, Fig III-15) must be considered as an artist’s schematic diagram of data obtained by Penfield and Jasper on many patients; it is not entirely accurate for each patient. However, the homunculus does show significant organization of the cerebral cortex in an anatomically meaningful way (see reference 85, p. 71, Fig. III-17).
Some body parts (e.g., the tongue and hand) have large cortical sensory areas. Picard and Olivier have extensively reviewed the anatomy of the sensory tongue cortex in humans. The language-dominant hemisphere had a larger sensory cortical tongue representation and the tip of the tongue had more extensive representation than the middle or back portions of the tongue.89 Most cortical representation was contralateral to somatic localization, but ipsilateral and bilateral responses to stimulation were reported, suggesting possible stimulation of the secondary sensory area, located at the base of the perirolandic cortex.
Penfield and Rasmussen88 found a human second sensory area in the region of the termination of the motor strip in the frontoparietal operculum (see reference 85, p. 79, Fig III-21). When stimulated, the second sensory area on the superior bank of the frontoparietal sylvian fissure produces bilateral, contralateral, or ipsilateral sensations, often of the perioral regions (see reference 85, p. 79, Fig III-21). Ipsilateral inputs are less numerous as shown by Adrian in cats.1 Lüders et al. recorded evoked potentials from the second sensory area that were of lower amplitude than primary sensory potentials.67
Other primary sensory cortices show highly organized anatomy; for example, the visual cortex deep within the calcarine fissure is organized so that the occipital pole is innervated polysynaptically by the macula and therefore is activated by the central visual field, whereas more rostral portions of the calcarine cortex contain representations of more peripheral visual fields.
The auditory cortex in the Heschl gyrus of the temporal lobe is tonotopically organized as shown by evoked potential mapping of longer-latency responses with magnetoencephal- ography.82,115,119,120 Vestibular cortex is also in the superior temporal gyrus rostral to the auditory cortex, but stimulation does not produce anatomically specific symptoms, just mild vertigo.85
Taste is represented in the parietal operculum and possibly near the insula,85 though Hausser-Hauw and Bancaud’s detailed depth electrode studies point primarily to the parietal operculum.43 Olfaction initially involves pathways from the olfactory epithelium through the cribriform plate to the olfactory bulbs, olfactory tracts, and lateral stria. These areas project to the anterior perforated space, prepiriform cortex, lateral olfactory gyrus, periamygdaloid cortex, entorhinal cortex, amygdala, septal nuclei, and hypothalamus.122 Thus, most olfactory centers are located in the mesial temporal region and adjacent structures.
While a description of epileptogenesis is beyond the scope of this chapter, clearly any structural lesion, whether seen by MRI or not, involving a sensory cortex or adjacent area may provide the necessary and sufficient changes for chronic localization-related epilepsy to develop. The concept of the epileptogenic zone, as interpreted by Penfield and Jasper85 and Ajmone-Marsan4 and reviewed by Lüders and Awad,65 implies that the locations in the cortex where symptoms are produced, where seizures are generated, where interictal spikes occur, and of any structural lesions all may contribute to epileptogenesis or to the symptom expression during seizures. It is certainly possible for sensory cortex to become activated late in a seizure and thus produce symptoms after the actual EEG onset in a silent cortical area. To experience somatosensory, visual, or auditory symptoms, white matter pathways, association cortices, and brain structures regulating attention and consciousness all play a role.
Epileptogenesis producing sensory seizures and focal epilepsy may occur without brain lesion or injury from peripheral nervous system injury. Spiller et al. reported three cases with soft tissue injuries to the hand where sensory seizures developed within months in the injured limb and all cases eventually had secondarily generalized tonic–clonic seizures. The authors postulated that the injury resulted in cortical reorganization that was epileptogenic.109
Clinical Features
Simple sensory seizures show a wide variety of phenomenology and represent some of the most interesting seizures reported. Patients can have prolonged symptoms70 and can relate elaborate descriptions of their seizure. Since sensory cortices are located in adjacent lobes, it is not surprising that multimodality sensory seizures are frequently reported in addition to sensory seizures from one modality.72
Seizures of the primary sensory cortex typically produce contralateral positive or negative symptomatology. Unilateral symptoms thought to originate in or near the contralateral postcentral gyrus include tingling, numbness, sense of movement, desire to move, somatic pain, heat or cold, electric shock sensations, agnosia for a body part, and phantom sensations.60,72,85,99,123,129 The hand and fingers are most often involved initially.72 Symptoms may be stationary or have a sensory march. Somatosensory seizures may be interpreted as motor activity by the patient, even when there is no visible movement seen. This can often lead to misinterpretation of seizure localization by a physician taking the history. Cephalic pain, occasionally migrainous in nature,19 even if unilateral, may not have localizing value, while abdominal pain usually originates from the temporal lobe.129 Genital pain probably originates at the mesial parietal termination of the sensory strip and is not necessarily associated with orgasmic seizures.98,110,128 Attacks of limb agnosia (sudden loss of sensation for a body part) and phantom limb sensations (sense that the limb is in a position that is not the true position) probably originate in the posterior parietal region.41,72,99 Ajmone-Marsan gives Foerster credit for pointing out that a postcentral-area seizure can be distinguished from the precentral seizure since motor symptoms stop earlier when the seizure originates in the postcentral gyrus and that the convulsing muscles are often more limited in distribution than when the primary motor cortex is seizing. Additionally, when a motor seizure occurs after activation of the sensory cortex, a tremor may be seen before true clonic spasms, if clonic spasms occur.4 Russell and Whitty observed that somatosensory auras often spread quickly to involve at least the entire limb and that prolonged sensory seizures, comparable to the epilepsia partialis continua of motor seizures, were not observed in their 85 cases. Motor seizures rarely progress to a simple somatosensory seizure, while the reverse is common. Sensory seizures often end abruptly, unlike the gradual cessation typical of focal motor attacks.99
Seizures from the second sensory area may produce ipsilateral or bilateral symptoms that are identical in character to symptoms occurring in the primary sensory cortex (e.g., numbness and tingling). Affected body parts can be diffuse or axial but often symptoms localize to the fingertips, feet, lips, or tongue.2,6,11,13,61,85,93,123 Second sensory-area seizures can arise from the frontoparietal operculum or the inferior parietal lobule.126 It is also possible to have ipsilateral sensations with seizures originating near the supplementary motor area since there may be a corresponding supplementary sensory region adjacent to the primary sensory foot area.85 Arseni and Maretsis’ case 3 may have had ipsilateral seizures from this location.6
Somatosensory auras have been reported in temporal lobe epilepsy, usually with bilateral or unilateral tingling. Pain and numbness were also noted in Erickson et al.’s report and most symptoms were in the limbs but could involve the head or trunk.36
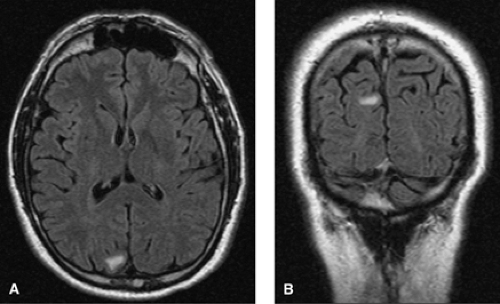
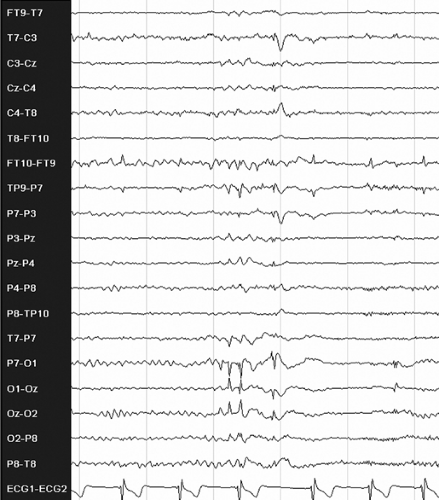 FIGURE 2. Interictal electroencephalogram showing left occipital spikes in an 18-year-old, right-handed male patient with a history of seizures since age 5, starting 3 months after a mild head trauma. He has an aura of right-sided flashing lights, then vertigo, followed by a subjective feeling of right arm twitching and then loss of consciousness. After that he turns his head to the right, raises his right arm, and progresses into a secondarily generalized tonic–clonic seizure. Magnetic resonance imaging of the brain was normal. The patient became seizure free after adding levetiracetam to his monotherapy carbamazepine regimen.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|