Neonatal Seizures
The variable terminology used in the literature when neonatal paroxysmal events (e.g., convulsions, seizures, epileptic seizures, nonepileptic seizures, muscular twitching, and motor automatisms) are discussed reflects the difficulty with recognizing and interpreting motor phenomena and autonomic signs in the newborn. Such difficulties may result either in the overdiagnosis of epileptic seizures or in the delay of the diagnosis of true “epileptic” attacks because not all paroxysmal neonatal events are epileptic seizures. The electroencephalographic (EEG) and clinical phenomena that characterize neonatal seizures differ from the usual epileptic patterns of older age. Typical absences, jacksonian attacks, and generalized tonic-clonic convulsions are not observed in neonates, although some of the rapidly migrating movements that occur in multifocal clonic seizures or in bouts of shudders and tremors may mimic motor seizures. The commonly atypical manifestations and most types of bizarre or unusual transient events in the neonatal period may be epileptic seizures, especially if these are stereotyped, insensitive to stimuli or restraint, and periodically recurring.
The differences between epileptic attacks in newborn babies and those in older children probably reflect the incomplete neuroanatomic and neurophysiologic development of the neonatal brain. Although virtually all neurons are in place by the time of birth, their axonal and dendritic processes and synaptic connections are still incompletely developed in the neonatal human brain (Levene et al., 2001; Volpe, 2001), and myelination is limited to a few pathways, which do not include the main hemispheric commissures. Changes in the receptors for and roles of both excitatory (glutamate and aspartate) and inhibitory (γ-aminobutyric acid [GABA]) circuits play a major role in the modulation of cortical excitability, and their differential rate of maturation with age may explain the changing susceptibility to epilepsy at various ages (Holmes, 1997; Johnston, 1996; Moshé, 1987) and the variability in the clinical expression of seizures. In particular, GABAA receptors seem to play an excitatory role at this age as a result of differences in the concentration of intracellular chloride in the immature brain (Cherubini et al., 1991; Ben-Ari et al., 1988). A detailed study of the mechanisms that are supposedly responsible for the generation and propagation of epileptic discharges in neonates is beyond the scope of this chapter. The absence of generalized tonic-clonic seizures (GTCSs) probably reflects both the lack of a sufficient degree of the cortical organization necessary to propagate and to sustain the electrical discharge and the failure of interhemispheric transmission resulting from commissural immaturity.
However, not all clinical phenomena in neonates have the same mechanisms (Mizrahi and Kellaway, 1998; Aicardi, 1991b; Camfield and Camfield, 1987; Kellaway and Mizrahi, 1987). Some are regularly associated with rhythmic EEG discharges that are clearly epileptic in nature, whereas others are unassociated or only inconsistently associated with paroxysmal EEG changes.
In the first group of seizures, which consist mainly but not exclusively of clonic events, the origin of the ictal discharges appears to have a cortical focus, and these may often be correlated with discrete cortical insults. Morphologically, the discharges are quite reminiscent of the epileptic events observed in older patients, leaving little doubt about their epileptic nature.
In the second group, which consists mainly of tonic and subtle seizures, the mechanism is uncertain; although some such seizures are associated with cortical epileptic discharges that are not picked up on the scalp, such an explanation is unlikely to apply in all cases. At least part of these are probably not epileptic; they may instead represent “release” phenomena caused by the liberation of the subcortical (especially brainstem) structures from cortical control, usually because of extensive cortical destruction or dysfunction (Kellaway and Mizrahi, 1990; Volpe, 1990; Camfield and Camfield, 1987).
Such an explanation for the second group is supported by the fact that “release” phenomena are often graded rather than being an all-or-none phenomena, and they can be elicited by stimulation and inhibited by restraint. In addition, they may demonstrate spatial and temporal summation, which is not observed in
true epileptic seizures. Similar features have been demonstrated in animal models of reflex physiology, which supports the suggestion that these clinical phenomena may actually be nonepileptic in origin, representing exaggerated reflex behaviors (Mizrahi and Kellaway, 1998). Alfonso et al. (2001) reported two neonates who presented with clinically similar episodes of sustained tonic posture of the right upper limb, which, in one, were considered as brainstem release phenomena on the basis of EEG and clinical data and, in the other, as epileptic seizures. Ictal single photon emission computed tomography (SPECT) scans showed focal cerebral hemisphere hyperperfusion only in the second case. According to the authors, different perfusion characteristics of two similar clinical events suggest a different mechanism, refuting the theory that brainstem release phenomena are due to EEG seizures that are not detectable by scalp or nasopharyngeal EEG.
true epileptic seizures. Similar features have been demonstrated in animal models of reflex physiology, which supports the suggestion that these clinical phenomena may actually be nonepileptic in origin, representing exaggerated reflex behaviors (Mizrahi and Kellaway, 1998). Alfonso et al. (2001) reported two neonates who presented with clinically similar episodes of sustained tonic posture of the right upper limb, which, in one, were considered as brainstem release phenomena on the basis of EEG and clinical data and, in the other, as epileptic seizures. Ictal single photon emission computed tomography (SPECT) scans showed focal cerebral hemisphere hyperperfusion only in the second case. According to the authors, different perfusion characteristics of two similar clinical events suggest a different mechanism, refuting the theory that brainstem release phenomena are due to EEG seizures that are not detectable by scalp or nasopharyngeal EEG.
A growing body of information in neonates and older children (Arzimanoglou et al., 1999; Arzimanoglou, 1996; Harvey et al., 1996) suggests that epileptic phenomena can also be generated at subcortical levels. The studies of Scher et al. (1993) and Weiner et al. (1991) also support the possible role of subcortical structures because those groups with EEG accompaniments for the subtle seizures were clinically similar to those without EEG accompaniments and both had similar neurologic outcomes. Studies in rats support this hypothesis (McCown and Breese, 1992).
The problem is further compounded by the frequent occurrence of electrical discharges without clinical manifestations (Glauser and Clancy, 1992; Connell et al., 1989; Clancy et al., 1988; Bridgers et al., 1986; Hellstrom-Westas et al., 1985). These may be much more common than electroclinical seizures. Although they may precede, follow, or alternate with electroclinical attacks, they also occur in isolation. The converse phenomenon of typical clinical seizures without electrical concomitants has also been reported (Herranz Tanarro et al., 1984).
The distinction between epileptic and nonepileptic seizures may be of practical significance for therapy and prognosis (Volpe, 1989; Lombroso, 1992, 1996). However, clinical differentiation of these two types of seizures may be extremely difficult or even impossible. Indeed, clinically identical seizures in the same patient may only occasionally be associated with epileptic paroxysms, and seizures occurring late in long-lasting series are sometimes unaccompanied by EEG discharges (Delgado-Escueta and Enrile-Bacsal, 1984; Dreyfus-Brisac and Curzi-Dascalova, 1979). Scher et al. (1989) and Scher and Painter (1990) found ictal paroxysms in only 8 of 18 newborn infants with clonic seizures, although most investigators have noted a closer correlation (Lombroso, 1992). This is even more likely in newborn infants whose brains may have been gravely injured and whose EEG background activities may be quite abnormal or diffusely depressed (Lombroso, 2002). EEG monitoring and, even better, video-EEG are desirable, even if they do not answer all the questions. Important clinical indications include the stereotypic nature of the events, as well as the absence of sensitivity to stimuli and to restraint, which are easily looked for and have considerable value (Mizrahi and Kellaway, 1998).
The etiologic context and global neurologic evaluation provide a solid and indispensable basis for decision making (Arzimanoglou and Aicardi, 2001). The issue of “epileptic” versus “nonepileptic” seizures has been debated extensively, and the interested reader can consult the pertinent articles mentioned earlier for the different views, as well as a recent review of the issue (Lombroso, 2002).
As a result of these diagnostic difficulties, the true incidence of neonatal seizures varies in different series from 0.15% to 1.4% (Aicardi, 1991b; Legido et al., 1988; Goldberg, 1983; Bergman et al., 1982). The reported incidence in the general population of neonates was 3.5 per 1,000 livebirths in a retrospective cohort study by Lanska et al. (1995) and 2.5 per 1,000 livebirths in the prospective study from Ronen and Penney (1995). The incidence probably varies with gestational age, with most cases being observed in full-term infants (Curtis et al., 1988; Bergman et al., 1983; Hopkins, 1972). However, preterm infants do appear in most series (Scher et al., 1988; Painter et al., 1986; Bergman et al., 1983; Radvanyi-Bouvet et al., 1981). Of 121 neonates with seizures that were reported by Dreyfus-Brisac et al. (1981), 40 were premature, with 19 being born before 33 weeks of gestation. The extremely high incidence rate (up to 23%) that Bergman et al. (1983) reported in premature infants is probably due in part to the frequency of nonepileptic events in this group (Mizrahi, 1987; Mizrahi and Kellaway, 1987). Stratification of data according to birth weight (Lanska et al., 1995) shows a much higher incidence of an estimated 57.5 cases per 1,000 livebirths in very-low-birth-weight (i.e., less than 1,500 g) infants. Scher et al. (1993) estimated that 2.3% of all infants cared for in an intensive care unit experienced seizures.
Intrauterine seizures have been suspected in several reports (Holmes, 1985; Bejsovec et al., 1967), and they have been directly observed by ultrasonography
(Du Plessi et al., 1993; Landy et al., 1989). Movements that the mothers later recognize as probably being convulsive in nature mainly occur in the last days or weeks of gestation. Pyridoxine dependency is another possible cause of intrauterine convulsions (Bejsovec et al., 1967; Mikati et al., 1991), but other causes, especially brain dysplasia (Du Plessis et al., 1993), are possible.
(Du Plessi et al., 1993; Landy et al., 1989). Movements that the mothers later recognize as probably being convulsive in nature mainly occur in the last days or weeks of gestation. Pyridoxine dependency is another possible cause of intrauterine convulsions (Bejsovec et al., 1967; Mikati et al., 1991), but other causes, especially brain dysplasia (Du Plessis et al., 1993), are possible.
This chapter first describes the clinical manifestations of neonatal seizures, including all stereotypic events in which no sensitivity to stimuli is present and which are not abolished by restraint. The relationship of the various clinical phenomena with EEG events is then considered, followed by a discussion of the diagnostic, etiologic, and treatment issues.
CLINICAL AND ELECTROENCEPHALOGRAPHIC FEATURES
The seizures of newborns require a different classification than those that are applied at other ages. Most authors recognize the following four main types of seizures: subtle (Volpe, 1989) or minimal, clonic, tonic, and myoclonic. Several seizure types often occur together in the same infant; subtle seizures are often associated with other types in severely ill neonates (Aicardi, 1991b). The possibility that some seizures may occur in the absence of simultaneous EEG seizure activity is widely accepted. The increasing use of EEG polygraphic video-monitoring techniques during the last decade has allowed a more precise description and classification of neonatal seizures and a better understanding of their pathophysiology.
Clonic Seizures
Clonic seizures consist of rhythmic muscle jerking that can involve any part of the body. The following two subtypes are recognized: focal clonic and multifocal. Unifocal seizures may affect one extremity or one side of the face; at times only a very limited territory may be influenced. Rarely, they can involve a whole side of the body. In some infants, the jerks remain confined to a limited muscle group or to the tongue, or they may involve the diaphragm or other axial muscles. In multifocal seizures, localized clonic movements shift, often quite rapidly, from the site of origin to another nonadjoining body part or to the opposite side in a disordered nonjacksonian fashion. Several segments can be involved simultaneously, but the jerking is not synchronous, even though the rapid shifting may superficially simulate a generalized seizure (Lombroso, 2002). The terms erratic (Lombroso, 1992; Volpe, 1990) and migratory have been applied to such seizures (Legido et al., 1988; Aicardi, 1985b). Such multifocal seizures probably have the same significance as focal clonic seizures as far as prognosis is concerned, but they do not indicate the presence of a fixed focal lesion, as is often true with unifocal seizures (Aicardi, 1991b). In neonates, these can, however, can be due to metabolic disturbances, and all variations of the spectrum between frank multifocal seizures and subtle seizures with minimal jerking and poor organization may be seen.
Unifocal seizures usually have good correlation with EEG activity, but they do not necessarily imply a focal pathology. Multifocal discharges usually accompany multifocal seizures. Careful direct observation and manipulation maneuvers (restraint, repositioning, stimulation) allow the seizures to be distinguished from manifestations seen in nonseizure states (Mizrahi and Kellaway, 1998; Lombroso, 2002), such as jitteriness, tremor, shudders, hypnic jerks, and benign myoclonus of sleep.
Subtle Seizures
The term subtle seizures was used to describe various behavioral phenomena that may involve the limbs, the axial muscles, or the face and eyes. Lip smacking, sucking or swallowing movements, mouth puckering, grimacing, eye deviations (lateral or vertical), repetitive blinking, and staring can all be observed. Abnormal limb movements may include complex patterns such as pedaling, boxing, swimming, or stepping or the assumption of a tonic posture by one limb or a segment thereof (Aicardi, 1991b; Volpe, 1990; Mizrahi, 1984; Rose and Lombroso, 1970). Autonomic phenomena, such as vasomotor changes, salivation, or modification of heart rate, may occur in an isolated fashion, but they are more often associated with motor automatisms. Several abnormal events are usually associated (Dreyfus-Brisac and Monod, 1972, 1977), and abnormal eye movements, especially in the horizontal plane (Blume, 1978), have a high diagnostic value. Subtle paroxysmal phenomena mostly occur in newborns with central nervous system insults (Lombroso, 2002).
Some authors (Mizrahi and Kellaway, 1998) prefer the term motor automatisms to describe these phenomena, which they usually consider nonepileptic in nature. Although this may be true for a number of cases, the use of video-EEG suggests caution when interpreting such phenomena. These motor behaviors or
automatisms may occur in premature or encephalopathic babies. When they are nonictal in nature, they are usually not accompanied by autonomic changes, they can be stopped by restraining or repositioning the child, or they can be triggered by stimulation. Drooling may, however, occur (Lombroso, 2002).
automatisms may occur in premature or encephalopathic babies. When they are nonictal in nature, they are usually not accompanied by autonomic changes, they can be stopped by restraining or repositioning the child, or they can be triggered by stimulation. Drooling may, however, occur (Lombroso, 2002).
Apneic seizures are common, and they rarely occur as the sole manifestation (Navelet et al., 1989; Fenichel, 1985; Watanabe et al., 1982). More often, they are seen in association with ocular or autonomic signs. Fenichel et al. (1980a) found that apneic seizures were not accompanied by bradycardia, while this was seen in the much more common nonepileptic apneas of the premature infant when these last 20 seconds or more. Central nonictal apnea is usually accompanied by agitated asynchronous movements of the limbs, whereas mainly oral automatisms, nystagmus, or eye deviation is observed with apneic ictal manifestations. Some apneas may be iatrogenic (Lombroso, 2002). Further investigations are needed to understand more fully the paroxysmal tachycardia, hypertension, or tachypnea that can be the sole manifestations of paroxysmal brain activity.
Tonic Seizures
Tonic seizures most often are generalized, featuring tonic extension of all limbs or, occasionally, flexion of the upper limbs with extension of the legs. These symmetric tonic postures are rarely true seizures. They more commonly represent “release” phenomena, and they can be triggered by stimulation. The background activity is usually abnormal, but ictal paroxysmal activity is not observed. However, abrupt tonic limb extension and/or flexion with abduction may represent true epileptic spasms (see Chapter 3).
Focal tonic seizures consist of sustained asymmetric posturing of one limb with flexion of the trunk toward the involved side. Tonic eye deviation may be associated with these. Autonomic phenomena, such as apnea, flushing, or mild cyanosis, are usually present. They are not affected by restraints nor are they triggered by stimulation. Eye signs, such as opening or closing movements of the eyelids, staring, or gaze deviation, or the occurrence of a few clonic jerks may be a clue to the epileptic mechanism. Ictal discharges may be of the delta, alpha, or beta-like types.
Myoclonic Seizures
Myoclonic seizures are uncommon. When they do occur, they can be erratic or fragmentary. More often, myoclonic jerks are generalized, and these may be associated with tonic spasms or multifocal clonic patterns. They often persist into infancy as more or less atypical infantile spasms (Lombroso, 1974, 1992). They may be provoked by stimulation, and, from a pathophysiologic point of view, they may or may not be epileptic. The overall neurologic context, which usually favors the presence of a severe neurologic insult, allows them to be distinguished quite easily from shudders or benign neonatal sleep myoclonus.
REPETITION OF SEIZURES
Isolated seizures are relatively uncommon in the neonatal period. The occurrence of at least a few attacks is the rule. In a significant proportion of the patients, the seizures continue for long periods.
Clancy and Legido (1987b) found that the average duration of 427 seizures in 42 newborns was 137 ± 11 seconds, with extremes of 10 seconds and 46 minutes. Most (97%) seizures lasted less than 9 minutes, and only 2 seizures lasted more than 30 minutes. Discharges occupied 22% of total recorded time. Presumably, the patients in this study were more severely affected than was the average infant with neonatal seizures, and patients with only a few seizures were underrepresented. The total duration of the seizure period varies from a few seizures up to several days or rarely weeks. However, neonatal seizures tend to be self-limited, and they generally last 24 to 96 hours (Camfield and Camfield, 1987; Bout et al., 1983; Cukier et al., 1976), another factor complicating the assessment of therapy.
The term status epilepticus in the neonatal period has been used by French investigators (Dreyfus-Brisac and Monod, 1972, 1977; Cukier et al., 1976; Monod et al., 1969) and Schulte (1966). Neonatal status epilepticus was defined by Dreyfus-Brisac and Monod (1977) and Monod et al. (1969) as the repetition of clinical and/or purely electrical seizures with the interictal persistence of an abnormal neurologic status. Using this definition, Dreyfus-Brisac et al. (1981) reported that, in one study, 79 of 121 newborns had status epilepticus rather than isolated convulsions. Cukier et al. (1976), redefining the term with greater precision, required the occurrence of electrical seizure discharges, each of which lasted for at least 10 seconds and was repeated for several hours in association with an abnormal neurologic state and unconsciousness. Clinical seizures may or may not be present. The latter definition does not include repeated clinical seizures without ictal EEG concomitants, as may occur in neonates who have been convulsing
for many hours (Dreyfus-Brisac et al., 1981; Dreyfus-Brisac and Monad, 1977).
for many hours (Dreyfus-Brisac et al., 1981; Dreyfus-Brisac and Monad, 1977).
The use of the term serial seizures is perhaps preferable to that of status epilepticus in the neonatal period because it does not refer to an abnormal interictal neurologic state, which may be impossible to assess reliably because of the interference of drug treatment. Whatever the repetition rate and duration of the seizure period, the occurrence of several seizure patterns in the same patient is the rule rather than the exception. With prolonged convulsive episodes, the individual seizures tend to progress from well-marked to poorly organized attacks both clinically and electrically (Dreyfus-Brisac and Monod, 1977).
ICTAL AND INTERICTAL ELECTROENCEPHALOGRAPHIC CORRELATES OF NEONATAL SEIZURES
The combination of both EEG and clinical criteria accurately diagnoses and classifies seizures in neonates. The interpretation must take into account the gestational age, medical history, and the results of clinical and laboratory examinations. The maturational aspects and the normal and abnormal features of neonatal EEG are beyond the scope of this chapter (Mizrahi and Kellaway, 1998; Hrachovy et al., 1990; Dreyfus-Brisac and Curzi-Dascalova, 1979).
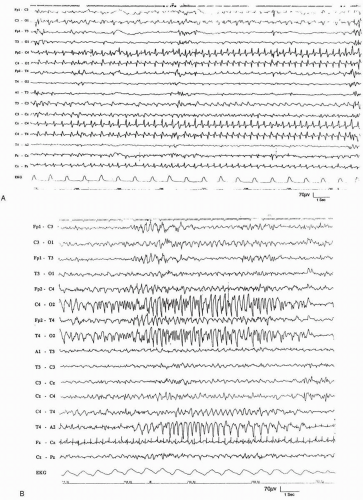
Except for the more generalized activity associated with myoclonic jerks or infantile spasms, almost all paroxysmal electrical activity in the neonate begins focally. Ictal discharges in the full-term neonate are exceedingly variable in appearance, voltage, frequency, and polarity (Rowe et al., 1985; Estivill et al., 1983; Dreyfus-Brisac, et al., 1981). Changes occur between discharges in the same infant and even within the same discharge. The modification in rhythm and polarity may be either progressive, usually with slowing of the rhythm toward the end of a discharge, or quite sudden, with abrupt changes in the frequency, morphology, and amplitude of the paroxysmal complexes (Fig. 12.1). Any schematic presentation is therefore inaccurate.
The following two main elements constitute the EEG discharges in newborns: abnormal paroxysmal rhythms and repetitive spikes or sharp waves. Both are commonly encountered (Estivill et al., 1983; Dreyfus-Brisac and Curzi-Dascalova, 1979; Dreyfus-Brisac, 1979; Harris and Tizard, 1960).
The abnormal ictal rhythms include alpha-like, thetalike, and delta-like rhythms (Fig. 12.1), with the last being most common (Willis and Gould, 1980; Dreyfus-Brisac, 1979). In a few patients, very fast (12 to 14 Hz) rhythms are recorded, especially at the onset of discharges. Fast rhythms, especially those in the beta range, may constitute the only abnormality in some seizures (Willis and Gould, 1980; Knauss et al., 1978). These are suggestive of a poor prognosis, and they are most often associated with other paroxysmal figures. Fast and slow rhythms are variably combined. They may occur in succession, with the fast rhythms usually coming first, or simultaneously, with the fast rhythms being superimposed on the delta rhythms. As a result of the combination of these various elements, the ictal EEG discharges in full-term babies are usually quite polymorphic (Tharp, 1981; Dreyfus-Brisac and Monod, 1977). The duration is quite variable. Most authors require a discharge to last at least 10 seconds to be considered ictal (Clancy et al., 1988; Radvanyi-Bouvet et al., 1985).
The focal discharges of sharp waves may be relatively fast (2 to 4 Hz), or they may occur at a slow (about 1 Hz) rate, a pattern resembling the periodic lateralized electrical discharges (PLEDs) of older patients (Holmes, 1985; Lombroso, 1982). The spikes or sharp waves are more frequently observed in the late part of the paroxysm, although they may represent the only abnormality. They are focal or multifocal, and they tend to involve the rolandic regions preferentially (Estivill et al., 1983; Dreyfus-Brisac et al., 1981). More often, they are associated with abnormal ictal rhythms, and, in combination with these rhythms, they may produce indented or notched diphasic or triphasic repetitive complexes (Estivill et al., 1983).
In premature infants, the EEG findings tend to be more stereotypic. Spikes are uncommon. They were observed in only 5 of 12 premature infants by Radvanyi-Bouvet et al. (1981, 1985), whereas spikes were present in 13 of the 15 full-term babies they studied. The most typical discharge in preterm infants is a delta rhythm with a steeply ascending initial deflection. Focal low-frequency discharges and multifocal spikes or sharp-wave rhythmic discharges are also observed (Rowe et al., 1985). The ictal discharges tend to be more synchronous over one hemisphere in premature infants than they are in full-term neonates. The duration of ictal discharges is quite variable, ranging from a few seconds to several minutes. In most patients, their diffusion remains quite restricted.
Some ictal patterns occur in neonates with severe encephalopathies; electrical seizures in the depressed brain are typically low in voltage, long in duration, and narrowly localized. These are usually seen in EEG recordings whose background activity is depressed and undifferentiated (Mizrahi and Kellaway, 1998).
RELATIONSHIP BETWEEN CLINICAL AND ELECTROENCEPHALOGRAPHIC ICTAL MANIFESTATION IN NEONATAL SEIZURES
Not all clinical phenomena traditionally regarded as seizures are regularly associated with EEG seizure discharges temporally (Legido et al., 1988; Camfield and Camfield, 1987; Fenichel, 1985; Mizrahi, 1984). In a video-EEG study of 420 neonates from 28 to 44 weeks of gestational age, 100 of whom had either clinical seizures or electrographic seizures that were unaccompanied by clinical seizures, Kellaway and Mizrahi (1990) found that all patients with focal or multifocal clonic seizures, all those with focal tonic seizures, some of those with generalized myoclonic seizures and apneic seizures, and only a few of those with generalized tonic and subtle seizures had consistent ictal EEG activity. The experience of other investigators has been largely similar. Fenichel et al. (1980a) recorded 52 episodes of subtle seizures in 15 neonates, only one of whom had accompanying EEG discharges; and only 4 of 23 infants with such seizures recorded by Scher and Painter (1990) had concomitant EEG discharges. Radvanyi-Bouvet et al. (1981) recorded ictal discharges in 50 premature infants with mostly subtle seizures, but whether the association between the clinical and EEG events in these patients was consistent is unclear.
Concluding that most subtle and generalized tonic seizures are not accompanied by EEG seizure discharges and that at least some of these may well have a “nonepileptic” mechanism seems safe, but the possible occurrence of epileptic discharges in any clinical seizure precludes reaching definitive conclusions.
Clonic seizures are mostly associated with well-formed, repetitive spike and sharp-wave discharges (Kellaway and Mizrahi, 1990; Mizrahi, 1984; Lombroso, 1982; Fenichel et al., 1980b). Abnormal paroxysmal rhythms tend to be present in seizures with less conspicuous clinical manifestations, such as focal tonic seizures (Aicardi, 1991b; Fenichel et al., 1980b), apneic attacks (Kellaway and Mizrahi, 1990), and subtle seizures (Holmes, 1985; Rowe et al., 1985; Fenichel et al., 1980b).
EEG seizure discharges without detectable clinical manifestations are common in neonates, as the practice of long-duration EEG monitoring has shown. This is especially common in infants receiving antiepileptic drugs, particularly phenobarbital, which often produces “uncoupling” of the clinical and EEG seizure manifestations (Clancy et al., 1988; Shewmon, 1983). An undefined proportion of newborns may have such discharges without ever having behavioral seizures. This was the case for 11 patients of Mizrahi and Kellaway (1987), and it has also been found in several other studies (Mizrahi, 1987; Kellaway and Hrachovy, 1983). Hellstrom-Westas et al. (1985) used a cerebral function monitor to detect seizure discharges in 87 neonates over several days in an intensive care unit. Fourteen infants had seizures that lasted an hour or more, and, of these, two never had detectable clinical seizures. Similar results were obtained by Eyre et al. (1983) and Bridgers et al. (1986) using cassette recording. In some of these patients, the absence of clinical manifestations was due to therapeutic paralysis, but the same results were then obtained in several nonparalyzed neonates. Connell et al. (1989) found EEG seizure activity in 55 (25%) of 275 full-term or preterm neonates, and clinical seizure manifestations were totally absent in 23 of these infants. Clancy et al. (1988) indicated that up to 79% of EEG discharges may not have clinical accompaniment. The frequency of silent seizures seemed especially high in infants who were receiving antiepileptic drugs.
Conversely, clinical seizures that are deemed to be epileptic but that have no typical EEG abnormalities are also encountered (Weiner et al., 1991; Dreyfus-Brisac and Monod, 1977; Chariton, 1975). Weiner et al. (1991) compared 33 newborns with both clinical and EEG seizures to 18 with only clinical seizure activity. They found that those without electrographic discharges had more interictal background EEG abnormalities and a higher frequency of subcortical lesions. Dreyfus-Brisac and Monod (1972, 1977) and Cukier et al. (1976) indicated that clinical seizures without EEG discharges were more common in cases in whom several seizures occurred after a long period of seizure activity (Clancy et al., 1986).
DIAGNOSIS OF NEONATAL SEIZURES
The most difficult issues in the diagnosis of neonatal seizures are deciding (a) which atypical ictal behavioral events can be regarded as epileptic seizures and (b) whether subclinical EEG seizures are occurring. For both purposes, EEG monitoring is essential, and this should occur over a prolonged period to answer the question regarding subclinical seizures (Lombroso, 1992; Scher and Painter, 1990; Scher and Beggarly, 1989; Legido et al., 1988).
The best methods of monitoring are still under discussion. Conventional multichannel EEG recording, cassette EEG monitors, cerebral function monitors, and compressed spectral array have all been used (Eaton et al., 1992). The essential point with the use
of any neurophysiologic technique is that the technician and doctor in charge should be familiar with the EEG of the newborn, which is quite different from that of adults (Lombroso, 1992; Plouin, 1990; Tharp, 1981). The issue of whether behavioral seizures that are unaccompanied by EEG discharges should be regarded as different in nature from electroclinical seizures or whether they represent only a variant of the same basic epileptic phenomenon has been discussed earlier in this chapter.
of any neurophysiologic technique is that the technician and doctor in charge should be familiar with the EEG of the newborn, which is quite different from that of adults (Lombroso, 1992; Plouin, 1990; Tharp, 1981). The issue of whether behavioral seizures that are unaccompanied by EEG discharges should be regarded as different in nature from electroclinical seizures or whether they represent only a variant of the same basic epileptic phenomenon has been discussed earlier in this chapter.
A number of abnormal phenomena in the neonatal period generally are not epileptic seizures, or they are only on rare occasions. Jitteriness, which is characterized by tremulous movements of a periodic nature that have equal speed in the alternating phases of flexion and extension, is usually easy to distinguish from convulsive jerks, which have a fast active phase and a slower release component. Jittery tremor is usually generalized to all four limbs, but they may be observed in a single limb or in a particular position of that limb; this is not accompanied by abnormal eye movements (Fernandez-Alvarez and Aicardi, 2001; Brown and Minns, 1980; Brown, 1973). Almost one-half of normal full-term neonates exhibit jitteriness during the first days of life, when they are excited or crying (Parker et al., 1990), but the phenomenon can also occur in infants with hypoxic-ischemic encephalopathy (HIE), hypocalcemia, hypoglycemia, and drug withdrawal (Volpe, 2001). Jitteriness and seizures can occur coincidentally, and they can be difficult to differentiate.
Shuddering episodes can also be observed in the first months of life. They consist of brief (5 to 15 seconds) bursts of rapid tremor of the head and arms that are reminiscent of a shiver (Pachatz et al., 1999; Vanasse et al., 1976). Such cases might be associated with those of “benign myoclonus of infants” (Lombroso and Fejerman, 1977).
Benign neonatal sleep myoclonus (Resnick et al., 1986) is characterized by clonic movements of the limbs that occur only during slow sleep. The phenomenon is less common or is absent during rapid eye movement (REM) sleep (Di Capua et al., 1993), and waking the infant up always stops the jerks. In most patients, the jerks predominate in the upper limbs, especially distally, and they may be bilateral or localized, rhythmic and arrhythmic, and even migratory or multifocal (Fernandez-Alvarez and Aicardi, 2001). They may occur in salvoes, mimicking clonic seizures or even status epilepticus (Alfonso et al., 1995) when they occur repetitively for 20 to 30 minutes or even up to 90 minutes. No associated interictal or ictal EEG abnormality is present. Benign neonatal sleep myoclonus fades spontaneously from the second month onward, and it usually disappears before the sixth month of life (Fernandez-Alvarez and Aicardi, 2001).
Motor automatisms (Mizrahi and Kellaway, 1998; Kellaway and Mizrahi, 1990; Fenichel, 1985) can include several types (see “Subtle Seizures” earlier in this chapter), and they are only rarely associated with EEG discharges. This is especially applicable to the pedaling and boxing movements observed in metabolic disorders, such as leucinosis or organic acidurias (Livet et al., 2002). The multifocal myoclonic jerks observed in nonketotic hyperglycinemia (Seppälainen and Similä, 1971) may or may not be associated with EEG bursts, and these are often accompanied by other more or less typical electroclinical seizures (Aicardi and Goutières, 1978). True convulsive seizures are late events in most neonatal metabolic disorders, except for in the neonatal hyperammonemias, in which they may be the presenting symptom (Livet et al., 2002).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree