Chapter 3 Neoplasms of the Brain
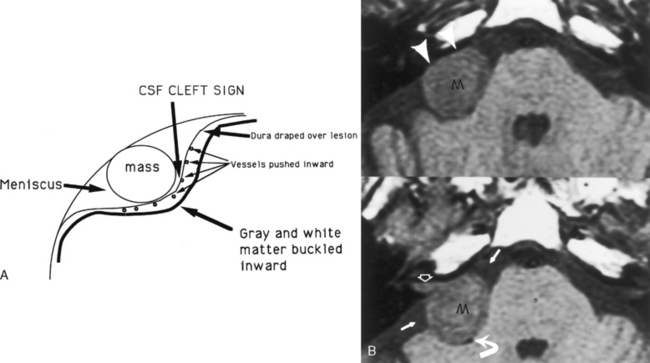
The essential distinction that a neuroradiologist must make is whether a lesion is intra-axial (intraparenchymal) or extra-axial (outside the brain substance; i.e., meningeal, dural, epidural, or intraventricular). This distinction has been made easier by the multiplanar capabilities of magnetic resonance (MR) imaging. The quintessential and most common extra-axial mass is the meningioma, a readily treatable and diagnosable lesion. The meningioma is not only extra-axial, but also intradural. Extra-axial intradural lesions buckle the white matter, expand the ipsilateral subarachnoid space, and sometimes cause reactive bony changes. On MR scans you can visualize the dural margin and determine that the lesion is extra-axial. The prototypical extradural (epidural) extra-axial mass, a bone metastasis, displaces the dura inward (is superficial to the dural coverings) but otherwise may have the same contour as an intradural extra-axial mass (Fig. 3-1).
EXTRA-AXIAL TUMORS
Tumors of the Meninges
Meningiomas
Meningiomas constitute the most common extra-axial neoplasm of the brain (Box 3-1). This lesion commonly affects middle-aged women. However, because of its incidence, it represents a significant proportion of the extra-axial neoplasms in men and in adults of all age groups. The most common locations for meningioma (in descending order) are the parasagittal dura, convexities, sphenoid wing, cerebellopontine angle cistern, olfactory groove, and planum sphenoidale. Ninety percent occur supratentorially. One percent of meningiomas occur outside the central nervous system (CNS), presumably from embryologic arachnoid rests. The most common sites for these “extradural” meningiomas are the sinonasal cavity, parotid gland, deep tissues, and skin. Because meningiomas arise from arachnoid cap cells, they can occur anywhere that arachnoid exists.
Box 3-1 WHO Classification of Tumors of the Meninges
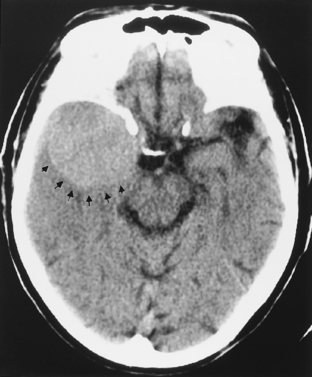
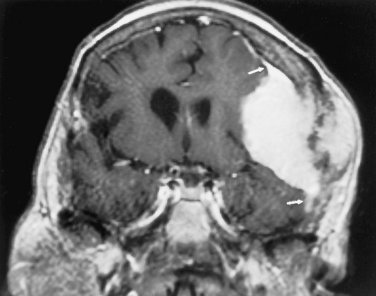
On unenhanced computed tomography (CT) scans, approximately 60% of meningiomas are slightly hyperdense compared with normal brain tissue (Fig. 3-2). You may see calcification within meningiomas in approximately 20% of cases. Rarely, cystic, osteoblastic, chondromatous, or fatty degeneration of meningiomas occurs. The specific histologic subtypes of meningioma—transitional, fibroblastic, and syncytial—cannot be readily distinguished on imaging.
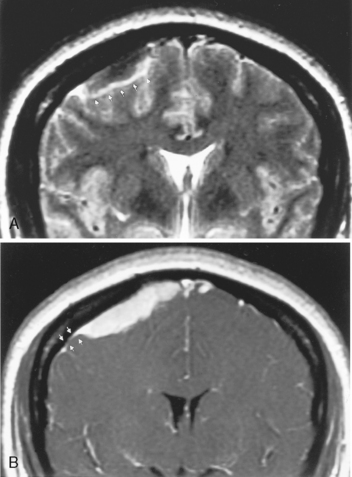
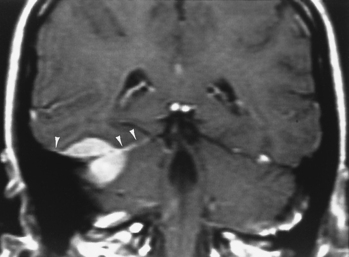
Magnetic resonance imaging is superior to CT in detecting the full extent of meningiomas, sinus invasion or thrombosis, vascularity, intracranial edema, and intraosseous extension. The typical MR signal intensity characteristics of meningiomas consist of isointensity to slight hypointensity relative to gray matter on the T1-weighted image (T1WI) and isointensity to hyperintensity relative to gray matter on the T2-weighted image (T2WI). The “cleft sign” has been described in MR to identify extra-axial intradural lesions such as meningiomas. The cleft usually contains one or more of the following: (1) cerebrospinal fluid (CSF) between the lesion and the underlying brain parenchyma, (2) hypointense dura (made of fibrous tissue), and (3) marginal blood vessels trapped between the lesion and the brain. One may see vascular flow voids on MR within and around a meningioma. Avid enhancement after contrast is also seen (Fig. 3-3), but occasionally meningiomas may have necrotic centers or calcified portions, which may not enhance. With MR you may be able to identify the dural tail, enhancement of the dura trailing off away from the lesion in crescentic fashion, which is typical of meningiomas and has been exhibited in up to 72% of cases (Fig. 3-4). There has been a debate in the radiologic literature as to whether the dural tail always represents neoplastic infiltration of the meninges by the meningioma or, alternatively, a reactive fibrovascular proliferation of the underlying meninges. In the typical situation where a differential diagnosis of vestibular schwannoma, meningioma, or fifth nerve schwannoma is debated, the dural tail may be a useful sign to suggest meningioma rather than other diagnoses (Table 3-1). The dural tail is not usually seen with schwannomas, although dural metastases may demonstrate a similar finding.
Table 3-1 Differential Diagnosis of Meningioma versus Schwannoma
| Feature | Meningioma | Schwannoma |
|---|---|---|
| Dural tail | Frequent | Extremely rare |
| Bony reaction | Osteolysis or hyperostosis | Rare |
| Angle made with dura | Obtuse | Acute |
| Calcification | 20% | Extremely rare |
| Cyst/necrosis formation | Rare | Up to 10% |
| Enhancement | Uniform | Inhomogeneous in 32% |
| Extension into the internal auditory canal | Rare | 80% |
| MRS | Alanine | Taurine, GABA |
| Precontrast CT attenuation | Hyperdense | Isodense |
| Hemorrhage | Rare | Somewhat more common |
CT, computed tomography; GABA, gamma-aminobutyric acid; MRS, magnetic resonance spectroscopy.
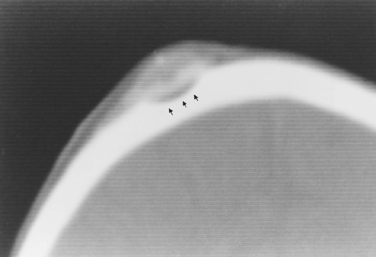
Intraosseous meningiomas may appear as expansions of the inner and outer tables of the calvarium or may even extend into the scalp soft tissues (Fig. 3-5). No dural component may be present at all. This type of meningioma strongly resembles a blastic osseous metastasis. Intraventricular meningiomas typically occur around the choroid plexus (80%) in the trigone of the lateral ventricle and have a distinct propensity for the left lateral ventricle (Fig. 3-6). Only 15% of intraventricular meningiomas occur in the third ventricle, and 5% occur in the fourth ventricle. Intraventricular meningiomas calcify in 45% to 68% of cases, and their frequency is higher in children. Multiple meningiomas are associated with neurofibromatosis type 2 (NF-2).
Meningiomas are one of the few tumors where angiography still may play an important role. Meningiomas diagnostically appear as lesions with an angiographic stain (tumor blush) and have both dural and pial blood supply. The characteristics of the stain are classically compared with an unwanted guest who comes early and stays late. Depending on the location of the tumor, you may have to perform internal carotid, external carotid, and vertebral injections (Table 3-2). In the typical convexity or sphenoid wing meningioma, the middle meningeal artery is enlarged (seen by primitive radiologists before angiography as enlarged meningeal grooves on the lateral skull film). At present preoperative embolization of meningiomas is sometimes performed to decrease the vascularity of the tumor. Make your referring neurosurgeon’s day by decreasing the intraoperative blood loss. Polyvinyl alcohol particles are most commonly used as the embolant (100 to 250 micromillimeters in size), but Gelfoam, cyanoacrylate, and trisacryl gelatin microspheres (Embospheres) are also employed by some.
Table 3-2 Blood Supply to Meningiomas
| Location of Meningioma | Commonly Seen Blood Supply (Origin of Vessel) |
|---|---|
| Convexity | |
| Sphenoid wing | Middle meningeal artery (ECA) |
| Tentorium and cerebellopontine angle | Tentorial artery (artery of Bernasconi-Casanari) from meningohypophyseal trunk (ICA) |
| Olfactory groove | Branches from ophthalmic artery |
| Foramen magnum and clivus | |
| Posterior fossa dura and falx cerebelli | Posterior meningeal artery (vertebral and MMA or ascending pharyngeal branches) |
ECA, external carotid artery; ICA, internal carotid artery; MMA, middle meningeal artery.
Radiation-induced Meningioma
The differential diagnosis of primary tumors that mimic meningiomas is broad (see Box 3-1).
Mesenchymal Meningeal Tumors
As noted in Box 3-1, there are a number of mesenchymal tumors that can affect the meninges. These are all relatively rare lesions, which may have either osseous (e.g., osteoma, osteosarcoma), chondroid (e.g., chondroma, chondrosarcoma), muscular (e.g., leiomyoma, rhabdomyosarcoma), fatty (e.g., lipoma, liposarcoma), or fibrous (e.g., fibroma, malignant fibrous histiocytoma) matrices associated with them. If you remember that the fibrous falx can be ossified (with bone and marrow fat), you can recall these tumors more readily.
Melanocytic Lesions
Within this category one finds diffuse melanocytosis, melanocytoma, neurocutaneous melanosis, and malignant melanoma. The melanin-containing cells are leftovers from neural crest origin. These diagnoses are difficult to make because metastatic melanoma to the dura does occur and must often be excluded. The melanocytoma is the most common of the lot and is seen in adults, whereas melanocytosis is more of a pediatric disorder (see Fig. 9-53). Although the latter is a diffuse process, the former usually presents as a posterior fossa mass. Hyperintensity on T1WI is the only hope for sealing this diagnosis, but the presence of this finding varies with melanin content. Spread of melanocytosis through the Virchow-Robin spaces is possible.
Tumors of Neurogenic Origin
Schwannomas
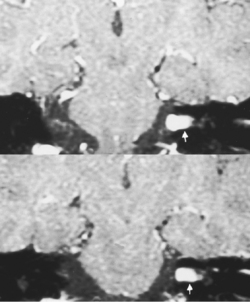
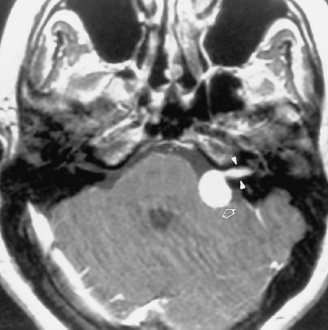
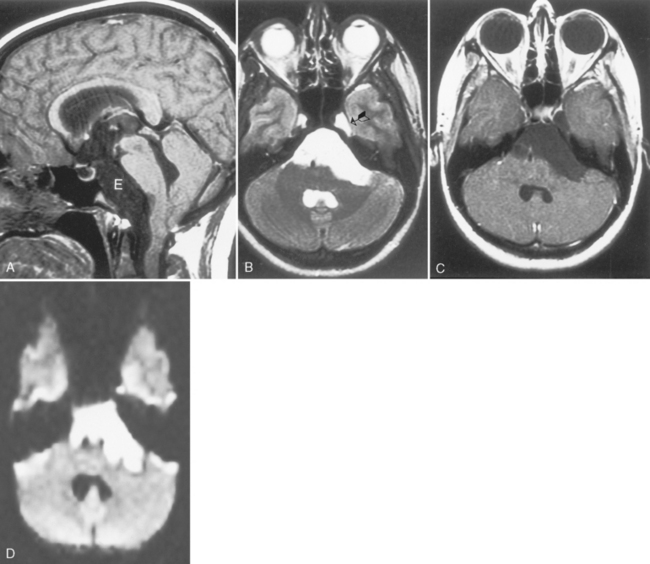
The distinction between meningiomas and schwannomas is a common one that radiologists must make (see Table 3-1). In some instances it is impossible to distinguish between the two. Both meningiomas and schwannomas may track along the course of the nerves; therefore the extent of the tumor is not helpful in distinguishing the two. As described previously, the dural tail is a helpful sign in suggesting a diagnosis of meningioma. In 81% of cases the border of a vestibular schwannoma makes an acute angle with the petrous bone; meningiomas usually make an obtuse angle. A curious finding has been described with vestibular schwannomas; arachnoid cysts coexist in 7% to 10% of cases, usually with the larger tumors (Fig. 3-7). These must be distinguished from cystic degeneration (and preponderance of Antoni B tissue) of the tumor, which is also a frequent phenomenon. Schwannomas may therefore be very bright on a T2WI, unusual for meningiomas. Vestibular schwannomas show microhemorrhages on susceptibility weighted scans in a high proportion—something absent in meningiomas.
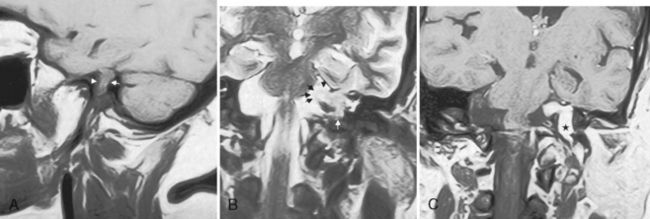
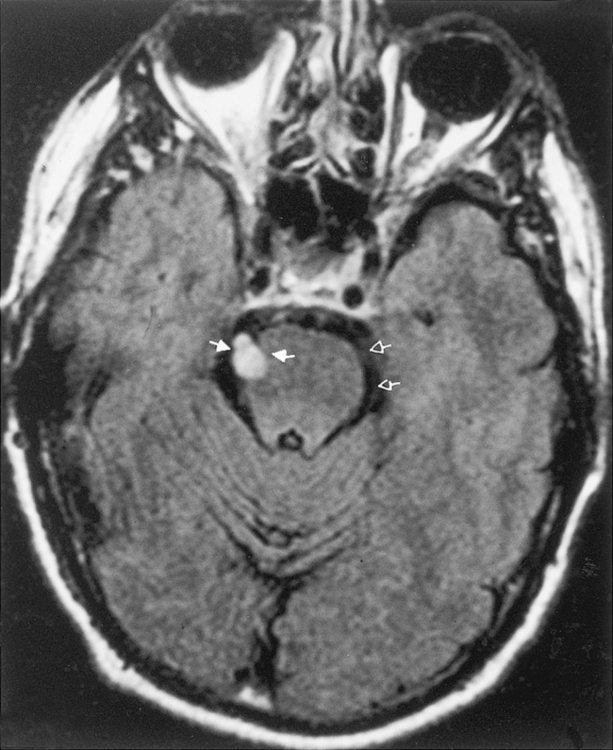
One of the features distinguishing vestibular schwannomas from meningiomas is the expansion of the internal auditory canal (IAC) seen in schwannomas. The porus acusticus (the bony opening of the IAC to the cerebellopontine angle cistern) is typically flared and enlarged with vestibular schwannomas, whereas the amount of tissue seen in the IAC with meningiomas is usually small or absent. Vestibular schwannomas account for more than 90% of purely intracanalicular lesions, but only 5% to 17% of them are solely intracanalicular (Fig. 3-8). Approximately 10% to 20% of vestibular schwannomas present only in the cerebellopontine angle cistern without an IAC stem (Box 3-2). Approximately 75% of vestibular schwannomas have a canalicular and cisternal portion (Fig. 3-9).
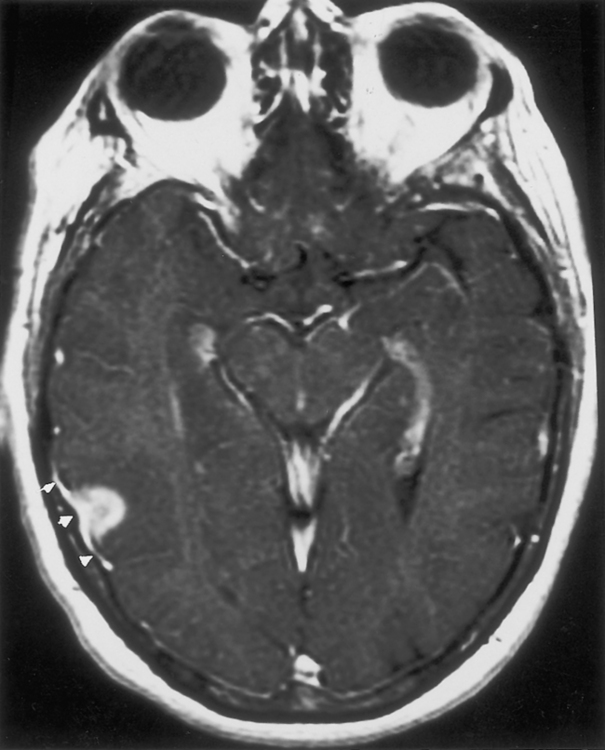
Schwannomas occur most commonly along cranial nerve VIII. The superior vestibular branch of cranial nerve VIII is the most common origin of the vestibular schwannoma (not “acoustic neuromas”), slightly more common than the inferior vestibular nerve. Nonetheless, patients typically have hearing loss. After lesions of cranial nerve VIII, schwannomas of cranial nerves VII (Fig. 3-10) and V are the most common site of intracranial neurogenic tumors, although ANY cranial nerve may be involved (Fig. 3-11).
Metastases
Dural Metastases
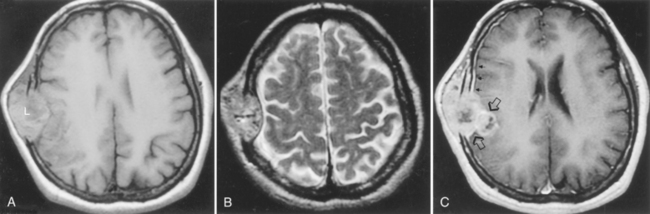
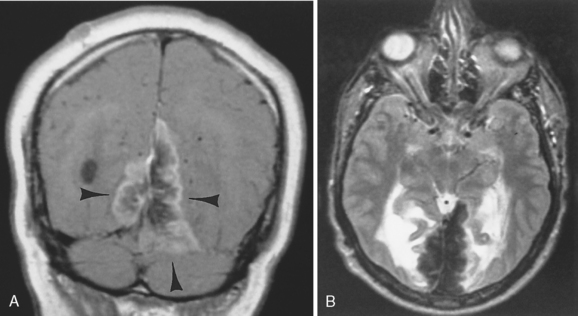
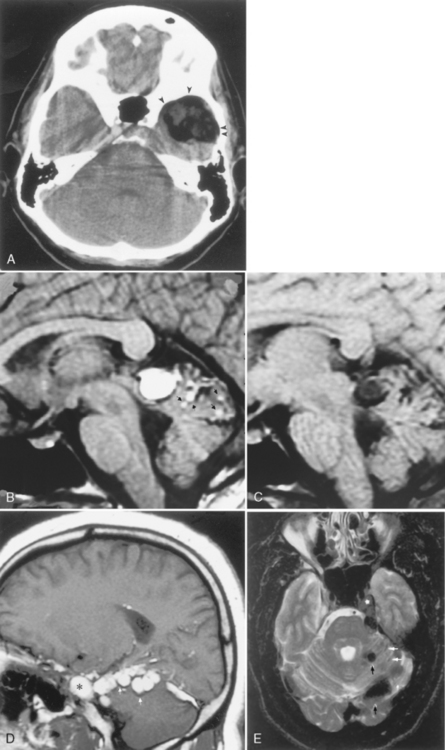
Dural metastases usually spread out along the dura as hematogenously disseminated en plaque lesions from extracranial primary tumors (Fig. 3-12). Lung, breast, and prostate cancer, as well as melanoma, are known to produce dural metastases. Some of the dural metastases may arise from spread of adjacent bone metastases. Breast carcinoma is the most common neoplasm to be associated with purely dural metastases. Lymphoma is next most common but is unique in that the dural lymphoma may be the primary focus of the neoplasm (Fig. 3-13). Dural plasmacytomas will look nearly identical to dural lymphoma (Fig. 3-14). In children dural metastases are most commonly associated with adrenal neuroblastomas and leukemia. These tumors are also famous for lodging in the cranial sutures, widening them in an infant.
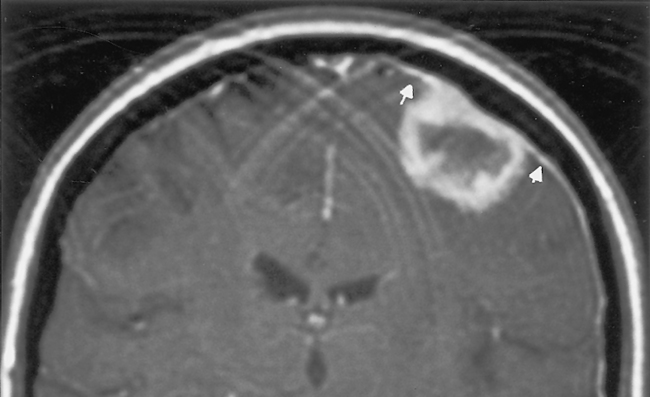
Occasionally, one can identify an adjacent parenchymal metastasis with dural spread (Fig. 3-15); alternatively, an osseous-dural metastasis (breast, prostate primaries) occasionally invades the parenchyma (Fig. 3-16). On MR the T1WI and T2WI characteristics are variable; however, typically the lesion is hypointense on T1WI and hyperintense on T2WI (Fig. 3-17 as an exception to the rule). On CT these lesions are identified as isodense thickening of the meninges. Contrast enhancement is prominent. This is a diagnosis where contrast-enhanced T1WI or fluid-attuenuated inversion recovery (FLAIR) scans can readily demonstrate the abnormality.
Subarachnoid Seeding
Usually one is dealing with tiny nodules of implanted tumor seedings. When the process is diffuse we use the term sugar-coating of the subarachnoid space because the whole pial surface is studded with sugar granules. A combination of sugar-coating and focal nodules may also occur. Subarachnoid seeding may occur with primary CNS tumors or primary tumors of other origins (Table 3-3). Lymphoma and leukemia are the most common tumors to seed the CSF. However, because they only rarely invade the meninges and do not incite reactions in the CNS, lymphomatous clusters are infrequently identified by neuroimaging techniques; the diagnosis is usually made by multiple spinal taps for CSF sampling. CSF seeding is associated with a mean survival of 1 to 2 months without and 6 to 10 months with treatment. The differential diagnosis could include arachnoiditis, Guillain-Barré syndrome, sarcoidosis, infectious granulomatous meningitides, Lyme disease, and cytomegalic inclusion virus (CMV) radiculitis.
Table 3-3 Sources of Subarachnoid Seeding
| CNS Primary | Non-CNS Primary |
|---|---|
| Children | |
| Medulloblastoma (PNET) | Leukemia/lymphoma |
| Ependymoma (blastoma = PNET) | Neuroblastoma |
| Pineal region tumors | |
| Malignant astrocytomas | |
| Retinoblastoma | |
| Choroid plexus papilloma | |
| Adults | |
| Glioblastoma multiforme | Leukemia/lymphoma |
| Primary CNS lymphoma | Breast |
| Oligodendroglioma | Lung |
| Melanoma | |
| Gastrointestinal | |
| Genitourinary | |
PNET, primitive neuroectodermal tumor.
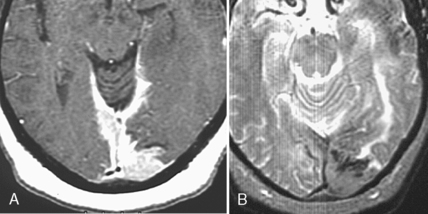
The typical locations where one identifies subarachnoid seeding are at the basal cisterns, in the interpeduncular cistern, at the cerebellopontine angle cistern, along the course of cranial nerves, and over the convexities (Fig. 3-18). One may see various manifestations of subarachnoid seeding, including the sugar-coated linear appearance to the enhancement of the surface of the brain (especially cerebellum) and spinal cord, or a nodular appearance of tumor deposits on nerve roots, both cranial and spinal. Often one identifies a peripheral intraparenchymal metastasis contiguous with the dural surface of the brain, from which cells are shed into the CSF. With subarachnoid seeding secondary hydrocephalus may be present.
Choroid Plexus Masses
Choroid Plexus Papilloma
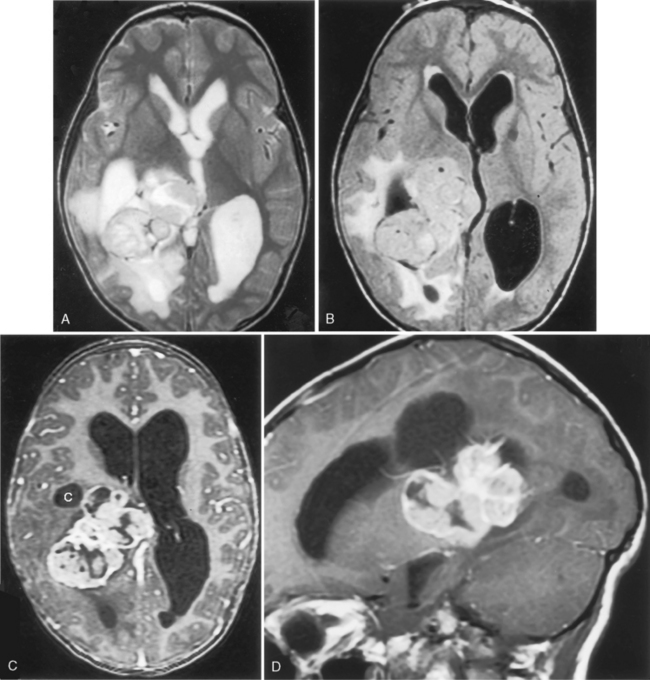
The tumors present with hydrocephalus and papilledema caused by overproduction of CSF (four to five times normal) or obstructive hydrocephalus caused by tumor, hemorrhage, high-protein CSF, or adhesions obstructing the ventricular outlets. Lately, the obstructionists have gained the majority from the overproductionists as far as the explanation for hydrocephalus. Calcification occurs in 20% to 25% of cases, and hemorrhage in the tumor is seen even more frequently than calcification. Choroid papillomas are typically hyperdense on unenhanced CT, with a mulberry appearance. Tumors are usually of low signal on T1WI and mixed intensity on T2WI unless hemorrhage has occurred. These tumors enhance dramatically (Fig. 3-19). Between the calcification, flow voids, and hemorrhage, the tumor has a heterogeneous appearance, sometimes with a salt-and-pepper appearance from vessel supply.
Choroid Plexus Hemangiomas
Choroid plexus hemangiomas are benign neoplasms of the choroid plexus usually seen in the lateral ventricle. Although the tumor enhances markedly and may calcify, it is usually seen as an incidental finding in an asymptomatic patient. There is an association with Sturge-Weber syndrome. In this syndrome, choroid plexus hemangiomas ipsilateral to the leptomeningeal vascular malformation may be present (see Chapter 9).
Choroid Plexus Xanthogranuloma
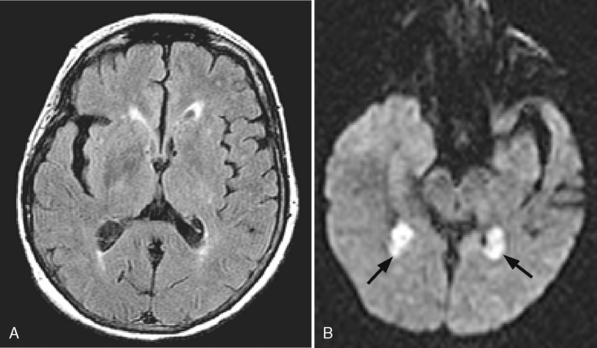
Another benign condition of the choroid plexus is the xanthogranuloma. This incidental lesion may have fat density/intensity within it and is also centered on the glomus of the trigone. Curiously, they are frequently bright on DWI scans (Fig. 3-20). They are of no clinical impact, only rarely causing visual disturbance. Box 3-3 lists common choroid plexus neoplasms. For choroid plexus inflammatory processes, consider Box 6-10.
Nonneoplastic Masses
Epidermoids
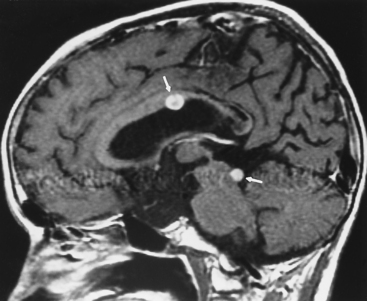
Epidermoids are collections of epithelium with desquamated debris (keratin and cholesterin) resulting from inclusion of ectodermal rests at the time of neural tube closure early in embryonic development. The walls are lined by simple stratified squamous epithelium, and the lesion has a pearly appearance. Men and women are affected equally, with a peak incidence in the 20- to 40-year age range. Epidermoids often occur in the cerebellopontine angle cistern (where they may present with trigeminal neuralgia and facial paralysis), the suprasellar cistern, the prepontine cistern, or the pineal region (Fig. 3-21). Extradural epidermoids are nine times less common than intradural ones and can arise within the diploic space, the petrous bone, and the temporal bone, where they appear as well-defined bony lesions with sclerotic borders (Fig. 3-22).
Epidermoids are typically of low density on CT. This is to be distinguished from dermoids or teratomas, which may have fat, bone, calcification, or other dermal appendages associated with them. Epidermoids expand to fill the interstices of the CSF space. An epidermoid is a lesion that is quite aggressive in insinuating itself around normal brain structures and often has scalloped borders. CT demonstrates a nonenhancing lobulated lesion. Sometimes epidermoids are hard to distinguish from arachnoid cysts, particularly in the cerebellopontine angle cistern (Table 3-4). Classically, epidermoids do not enhance. A stereotypical appearance on CT is that of displacement of the brain stem posteriorly by what appears to be just a dilated cistern anteriorly (see Fig. 3-21). In fact, this cistern is really a CSF density epidermoid with mass effect.
Table 3-4 Differentiation of Epidermoid and Arachnoid Cyst
| Characteristic | Arachnoid Cyst | Epidermoid |
|---|---|---|
| CT density | CSF | Slightly higher than CSF |
| Margins | Smooth | Scalloped |
| Calcification | No | 25% |
| Blood vessel involvement | Deviates | Insinuates between vessels |
| Intrathecal contrast | May take up but can be delayed | No uptake, defines borders |
| Characteristic on MR sequence sensitive to CSF pulsation(steady-state free precession) | Pulsates | Does not pulsate |
| Diffusion | Dark | Bright |
| ADC | Increased | Decreased |
| FLAIR | Dark | Bright |
ADC, apparent diffusion coefficient; CSF, cerebrospinal fluid; CT, computed tomo-graphy; FLAIR, fluid-attenuated inversion recovery; MR, magnetic resonance.
Dermoid
The high intensity of fat and signal void of calcification on T1WI suggests a diagnosis of a dermoid or teratoma (Fig. 3-23). Dermal appendages, such as hair follicles, sebaceous glands, and sweat glands, are found histologically in dermoid cysts. These lesions more typically occur in the midline as opposed to epidermoids, which are generally off the midline. Male patients are more commonly affected, and patients are younger than those with epidermoids. The presence of fat may be suggested by an MR chemical shift artifact seen as a hyperintense and hypointense rim at the borders of the lesion in the frequency-encoding direction. Fat suppression scans decrease the intensity on T1WI (see Fig. 3-23C). The possibility of a ruptured dermoid should be considered when multiple fat particles are seen scattered on an MR or when lipid is detected in the CSF. (Rule out Pantopaque droplets.) Usually the lesions are very well defined.
Lipoma
Lipomas also probably should not be placed in a chapter on neoplasms because they are most frequently developmental or congenital abnormalities associated with abnormal development of the meninx primitiva, a derivative of the neural crest. Lipomas are particularly common in association with agenesis of the corpus callosum, and 60% are associated with some type of congenital anomaly of the associated neural elements (see Fig. 9-19). The most common intracranial sites for lipomas are the pericallosal region, the quadrigeminal plate cistern, the suprasellar cistern, and the cerebellopontine angle cistern. Because the tissue is histologically normal but located in an abnormal site, lipomas should best be termed choristomas, not neoplasms. Fat defines the lipoma; look for low density on CT, high intensity on T1WI, and low intensity on conventional T2WI that suppresses even further when fat suppression techniques are applied.
INTRA-AXIAL TUMORS
Astrocytoma
The grading of astrocytomas by pathology groups is variable, but the latest WHO classification separates astrocytomas into circumscribed astrocytomas (grade I), diffuse astrocytomas (grade II), anaplastic astrocytomas (grade III), and GBM (grade IV) on the basis of histologic criteria (Box 3-4) and gross/imaging appearance. Circumscribed lesions include the pilocytic astrocytomas, subependymal giant cell astrocytomas, and pleomorphic xanthoastrocytoma (PXA). Fibrillary, gemistocytic, and protoplasmic astrocytomas are classified as diffuse grade II tumors. Then come the nasties—anaplastic astrocytoma and GBM—that are of the highest grade. Syndromes associated with astrocytomas are listed in Box 3-5.
Grade I: Circumscribed Astrocytomas
Juvenile Pilocytic Astrocytoma
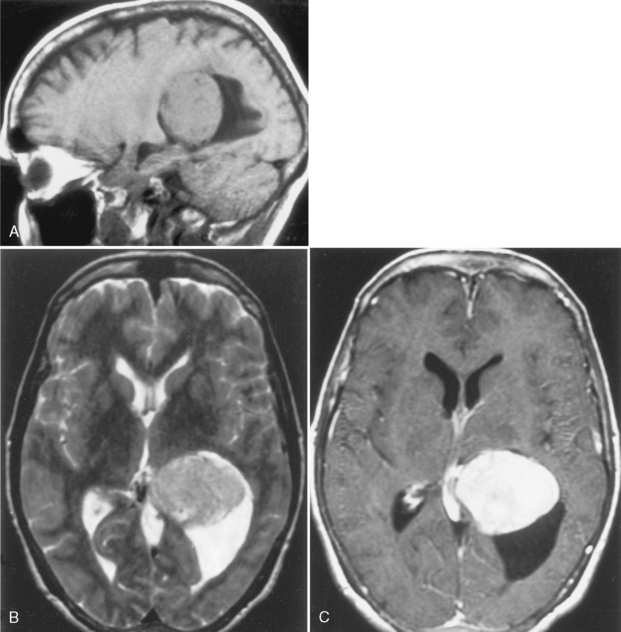
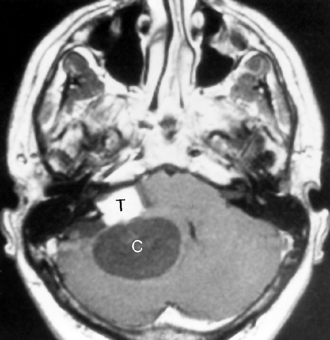
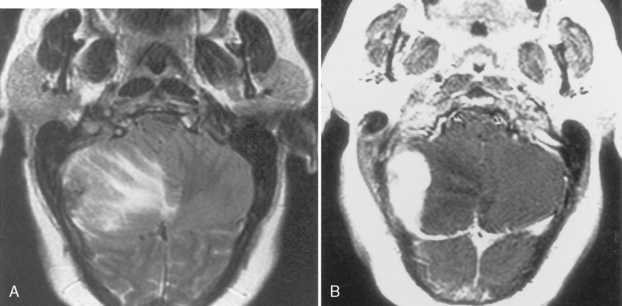
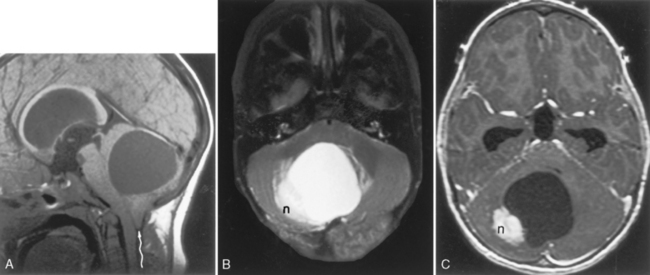
Cerebellar juvenile pilocytic astrocytomas (JPAs) are the most common infratentorial neoplasm in the pediatric age group and are classified as a WHO grade I astrocytic tumor (Table 3-5). They are seen in children. They are benign. They have a solid central piece and a separate peripheral portion. In general, pilocytic astrocytomas are well outlined from normal brain, are usually round, and usually are not ominous in appearance (Fig. 3-24). The lesions, when removed completely, are associated with an excellent prognosis (5-year survival rate >90%). Sixty percent of pilocytic astrocytomas occur in the posterior fossa, but they also favor the optic pathways and hypothalamus. Anaplasia is less common when the tumor is cystic than solid; therefore, the prognosis varies according to tumor morphology. The typical cerebellar astrocytoma in the pediatric age group is cystic (60% to 80%), whereas in older patients it is more likely to be solid. A mural nodule may be present with a similar appearance to the hemangioblastomas of adults. Occasionally the astrocytomas in the posterior fossa are solid, without a cystic component, and may simulate other pediatric posterior fossa masses (Table 3-6).
Table 3-5 WHO Classification of Astrocytic Tumors
| Tumor | Grade | Peak Age (years) |
|---|---|---|
| Pilocytic astrocytoma | I | 0–20 |
| Subependymal giant cell astrocytoma | I | 10–20 |
| Pleomorphic xanthoastrocytoma | II | 10–20 |
| Diffuse astrocytoma | II | 30–40 |
| Fibrillary | II | 30–40 |
| Protoplasmic | II | 30–40 |
| Gemistocytic | II | 30–40 |
| Anaplastic astrocytoma | III | 35–50 |
| Glioblastoma | IV | 50–70 |
| Giant cell glioblastoma | IV | 40–50 |
| Gliosarcoma | IV | 50–70 |
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree