15 Neoplastic Meningitis
Introduction
Neoplastic meningitis (NM) is the result of seeding of the leptomeninges by malignant cells. NM is not infrequent, and is becoming more common as cancer patients live longer.1–5 NM results in significant morbidity, and median survival is short despite therapy. However, treatment can result in significant palliation of the CNS and is best approached by a multidisciplinary team.
Epidemiology
Neoplastic meningitis is diagnosed in 1% to 5% of patients with solid tumors (in which case it is termed carcinomatous meningitis), 5% to 15% of patients with leukemia (termed leukemic meningitis) and lymphoma (termed lymphomatous meningitis), and 1% to 2% of patients with primary brain tumors.5 Autopsy studies show that 19% of patients with cancer and neurologic signs and symptoms have evidence of meningeal involvement.6 Adenocarcinoma is the most frequent histology, and breast, lung, and melanoma are the most common primary sites to metastasize to the leptomeninges.3,7,8 Although small cell lung cancer and melanoma have the highest rates of spread to the leptomeninges (11% and 20%, respectively), because of the higher incidence of breast cancer (with a 5% rate of spread), the later accounts for most cases in large series of the disorder.1,7,9–11 Carcinomas of unknown primary constitute 1% to 7% of all cases of NM.7
NM usually presents in patients with widely disseminated and progressive systemic cancer (>70%) but it can present after a disease-free interval (20%) and may even be the first manifestation of cancer (5% to 10%), occasionally in the absence of other evidence of systemic disease.7,12–14
Pathogenesis
Cancer cells reach the meninges by various routes: (1) hematogenous spread, either through the venous plexus of Batson or by arterial dissemination; (2) direct extension from contiguous tumor deposits; (3) and through centripetal migration from systemic tumors along perineural or perivascular spaces.15–18
Once cancer cells have entered the subarachnoid space, cancer cells are transported by CSF flow, resulting in disseminated and multifocal neuraxis seeding of the leptomeninges. Tumor infiltration is most prominent at the base of the brain, the dorsal surface of the spinal cord, and, in particular, the cauda equina.5,18 Hydrocephalus or impairment of CSF flow may occur at any level of the neuraxis and is due to ependymal nodules or tumor deposits obstructing CSF outflow.
Clinical Features
The most common manifestations of cerebral hemisphere dysfunction are headache and mental status changes. Other signs include confusion, cognitive impairment, seizures and hemiparesis. Diplopia is the most common symptom of cranial nerve dysfunction with cranial nerve VI being the most frequently affected, followed by cranial nerves III and IV. Trigeminal sensory or motor loss, cochlear dysfunction and optic neuropathy are also common findings. Spinal signs and symptoms include weakness (lower extremities more often than upper), dermatomal or segmental sensory loss, and pain in the neck, back, or following radicular patterns. Nuchal rigidity is only present in 15% of cases.3,7,8,13,19
New neurological signs and symptoms may represent progression of NM but must be distinguished from the manifestations of parenchymal disease (30% to 40% of patients with NM will have coexistent parenchymal brain metastases), from side effects of chemotherapy or radiation used for treatment, and, rarely, from paraneoplastic syndromes. At presentation, NM must also be differentiated from chronic meningitis due to tuberculosis, fungal infection or sarcoidosis, as well as from metabolic and toxic encephalopathies in the appropriate clinical setting.7,20
Diagnosis
CSF EXAMINATION
The most useful laboratory test in the diagnosis of NM is the CSF examination. Abnormalities include increased opening pressure (>200 mm H2O), increased leukocytes (>4/mm3), elevated protein (>50 mg/dl) or decreased glucose (<60 mg/dl), which though suggestive of NM are not diagnostic. The presence of malignant cells in the CSF is diagnostic of NM, but in general, as is true for most cytological analysis, assignment to a particular tumor is not possible.21
In patients with positive CSF cytology (discussed later), up to 45% will be cytologically negative on initial examination.6 The yield is increased to 80% with a second CSF examination, but little benefit is obtained from repeat lumbar punctures after the second.7 Of note, a series by Kaplan, including lymphomatous and leukemic meningitis, observed the frequent dissociation between CSF cell count and malignant cytology (29% of cytologically-positive CSF had concurrent CSF counts of less than 4/mm3).3 Murray showed that CSF levels of protein, glucose, and malignant cells vary at different levels of the neuraxis, even if there is no obstruction of the CSF flow.22,23 This finding reflects the multifocal nature of neoplastic meningitis and explains that CSF obtained from a site distant to that of the pathologically-involved meninges may yield a negative cytology.
Of the 90 patients reported by Wasserstrom, 5% had positive CSF cytology only from either the ventricles or cisterna magna.7 In a series of 60 patients with NM, positive lumbar CSF cytology at diagnosis, and no evidence of CSF flow obstruction, ventricular and lumbar cytologies obtained simultaneously were discordant in 30% of cases.24 The authors observed that in the presence of spinal signs or symptoms, the lumbar CSF was more likely to be positive and, conversely, in the presence of cranial signs or symptoms, the ventricular CSF was more likely to be positive. Not obtaining CSF from a site of symptomatic or radiographically demonstrated disease was found, in a prospective evaluation of 39 patients, to correlate with false-negative cytology results, as did withdrawing small CSF volumes (<10.5 ml), delaying processing of specimens, and obtaining less than two samples.25 Even after correcting for these factors, there remains a substantial group of patients with NM who have persistently negative CSF cytology. Glass reported on a postmortem study of the value of premortem CSF cytology,6 and demonstrated that up to 40% of patients with clinically suspected NM proven at the time of autopsy are cytologically negative. This figure increased to over 50% in patients with focal NM.
Numerous biochemical markers have been evaluated but, in general, their use has been limited by poor sensitivity and specificity. Particular tumor markers, such as CEA (carcinoembryogenic antigen) from adenocarcinomas, and AFP (α-fetoprotein) and β-HCG (β-human chorionic gonadotropin) from testicular cancers and primary extragonadal CNS tumors, can be relatively specific for NM when elevated in CSF in the absence of markedly elevated serum levels.16,26 Nonspecific tumor markers such as CK-BB (creatine-kinase BB isoenzyme), TPA (tissue polypeptide antigen, β2microglobulin, β-glucoronidase, LDH isoenzyme-5 and more recently VEGF (vascular endothelial growth factor) can be strong indirect indicators of NM, but none are sensitive enough to improve the cytological diagnosis.27–31 The use of these biochemical markers can be helpful as adjunctive diagnostic tests and, when followed serially, to assess response to treatment. Occasionally, in patients with clinically suspected NM and negative CSF cytology, they may support the diagnosis of NM.32
Use of monoclonal antibodies for immunohistochemical analysis in NM does not significantly increase the sensitivity of cytology alone.33–35 However, in the case of leukemia and lymphoma, antibodies against surface markers can be used to distinguish between reactive and neoplastic lymphocytes in the CSF.36
Cytogenetic studies have also been evaluated in an attempt to improve the diagnostic accuracy of NM. Flow cytometry and DNA single cell cytometry, techniques that measure the chromosomal content of cells, and fluorescent in situ hybridization (FISH), that detects numerical and structural genetic aberrations as a sign of malignancy, can give additional diagnostic information, but still have a low sensitivity.37–39 Polymerase-chain reaction (PCR) can establish a correct diagnosis when cytology is inconclusive, but the genetic alteration of the neoplasia must be known for it to be amplified with this technique, and this is generally not the case, particularly in solid tumors.40
In cases where there is no manifestation of systemic cancer and CSF examinations remain inconclusive, a meningeal biopsy may be diagnostic. The yield of this test increases if the biopsy is taken from an enhancing region on MRI (see below).41
NEURORADIOGRAPHIC STUDIES
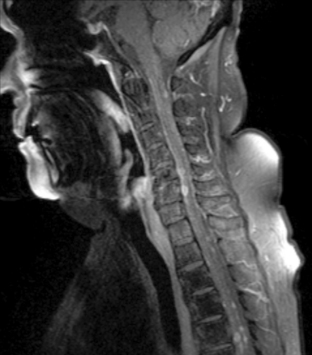
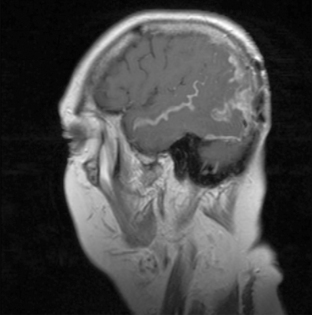
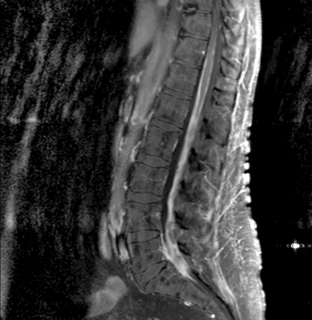
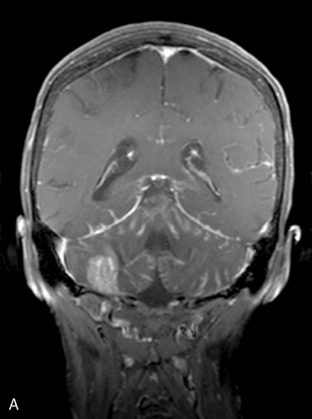
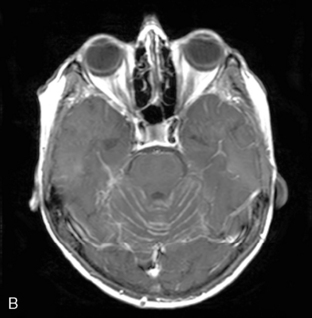
Magnetic resonance imaging with gadolinium enhancement (MR-Gd) is the technique of choice to evaluate patients with suspected NM (Figures 15-1 through 15-4).42–44 Because NM involves the entire neuraxis, imaging of the entire CNS is required in patients considered for further treatment. T1-weighted sequences, with and without contrast, combined with fat-suppression T2-weighted sequences constitute the standard examination.42–45 MRI has been shown to have a higher sensitivity than cranial contrast-enhanced computed tomography (CE-CT) in several series, and is similar to computerized tomographic myelography (CT-M) for the evaluation of the spine, but significantly better tolerated.42,44–46
Any irritation of the leptomeninges (i.e., subarachnoid blood) will result in their enhancement on MRI, which is seen as a fine signal-intense layer that follows the gyri and superficial sulci. Subependymal involvement of the ventricles often results in ventricular enhancement. Some changes, such as cranial nerve enhancement on cranial imaging and intradural extramedullary enhancing nodules on spinal MR (most frequently seen in the cauda equina), can be considered diagnostic of NM in patients with cancer.47 Lumbar puncture itself can rarely cause a meningeal reaction leading to dural-arachnoidal enhancement, so imaging should, preferably, be obtained prior to the procedure.48 MR-Gd still has a ≥30% incidence of false-negative results, so a normal study does not exclude the diagnosis of NM. On the other hand, in cases with a typical clinical presentation, abnormal MR-Gd alone is adequate to establish the diagnosis of NM.32,42,46,47
Radionuclide studies, using either 111Indium-diethylenetriamine pentaacetic acid or 99Tc macro-aggregated albumin, constitute the technique of choice to evaluate CSF flow dynamics.17,49 Abnormal CSF circulation has been demonstrated in 30% to 70% of patients with NM, with blocks commonly occurring at the skull base, the spinal canal, and over the cerebral convexities.46,49,50 Patients with interruption of CSF flow demonstrated by radionuclide ventriculography have been shown, in three clinical series, to have decreased survival when compared to those with normal CSF flow.49,51,52 Involved-field radiotherapy to the site of CSF flow obstruction restores flow in 30% of patients with spinal disease and 50% of patients with intracranial disease.53,54 Reestablishment of CSF flow with involved-field radiotherapy followed by intrathecal chemotherapy led to longer survival, lower rates of treatment-related morbidity, and a lower rate of death from progressive NM, compared to the group that had persistent CSF blocks.49,51
Prognosis
The median survival of untreated patients with NM is 4 to 6 weeks; death generally occurs due to progressive neurological dysfunction.7 Treatment is intended to improve or stabilize the neurological status, maintain neurological quality of life, and prolong survival. Fixed neurological defects are rarely improved with treatment, but progression of neurological deterioration may be halted in some patients and median survival can be increased to 4 to 6 months.16 Of the solid tumors, breast cancer responds best, with median survivals of 6 months and 11% to 25% 1-year survivals.26,55 Numerous prognostic factors for survival and response have been studied (age, gender, duration of signs of NM, increased protein or low glucose in CSF, ratio of lumbar/ventricular CEA, etc.), but many remain controversial.55 It is commonly accepted, however, that patients will do poorly with intensive treatment of NM if they have poor performance status, multiple fixed neurologic deficits, bulky CNS disease, coexistent carcinomatous encephalopathy, and CSF flow abnormalities demonstrated by radionuclide ventriculography. In general, patients with widely metastatic aggressive cancers that do not respond well to systemic chemotherapies are also less likely to benefit from intensive therapy.56,57 What appears clear is that, optimally, NM should be diagnosed in the early stages of disease to prevent progression of disabling neurological deficits, analogous to the clinical situation of epidural spinal cord compression.