Nervous System Complications of Cancer
Jack M. Rozental
Jeffrey J. Raizer
Neurologic complications of cancer can be metastatic, treatment related, or remote (paraneoplastic). They can cause neurologic disability or death, even while systemic disease is under control. Timely recognition and management of some complications can have a beneficial effect on quality and length of life. In most instances, management of nervous system complications is palliative. Thus, quality-of-life judgments may be more important than longevity in making therapeutic decisions.
I. METASTASIS TO THE BRAIN PARENCHYMA
A. About 20% of cancer patients develop metastases to the CNS, but only one half are symptomatic. Metastases are the most common CNS tumors (approximately 170,000 per year).
1. Seventy-five percent of brain metastasis are from lung (50%), breast (15%), and melanoma (10%); gastrointestinal, gynecologic, urologic, and cancers of unknown primary cause another 10%.
2. Twenty-five percent of metastatic lesions are single; about 20% of patients have two metastatic lesions.
3. Patients with gastrointestinal, gynecologic, or urologic tumors tend to have single brain lesions; approximately 50% are to the posterior fossa. Only 10% of other tumors metastasize to the posterior fossa.
4. Patients with lung cancer, melanoma, and tumors of unidentified origin usually have multiple metastatic lesions.
5. Most metastases localize to the frontal and parietal lobes (because of their relative mass and higher blood flow) and a few (1%) to the brainstem. Metastases lodge at the arterial border zones of the major cerebral vessels because of decreased vascular caliber and flow; hence, their preference for the gray-white junction.
6. The tumors most likely to bleed are melanoma, renal, and thyroid—the most vascular.
B. In up to 10%, an intracranial mass in patients with cancer is not a metastasis. The differential diagnosis includes:
1. Primary CNS tumor.
2. Brain abscess.
3. Demyelinating plaque.
4. Arteriovenous malformation.
C. Management.
1. Increased intracranial pressure (ICP) from a metastasis is usually managed with a bolus of dexamethasone 10 mg intravenously (IV) followed by 16 mg IV or orally in divided doses for maintenance.
2. For patients with minimal neurologic deficit no bolus is needed
3. If needed, dexamethasone is increased by 8 or more mg IV or orally four times a day.
4. Clinical improvement should be apparent within 24 to 48 hours of treatment, continue for several days, and then plateau.
5. Dexamethasone is tapered as tolerated after the patient’s condition is stable and more definitive therapy has started. The goal is to maintain optimal neurologic function on the lowest steroid dose due to the large number of side effects.
6. A proton pump inhibitor or H2 blocker may be started as prophylaxis against gastric bleeding, ulceration, or perforation.
D. Patients in extremis from increased ICP may need an osmotic diuretic, e.g., mannitol 20% solution 1 g per kg IV.
1. Smaller doses of mannitol (0.25 to 0.5 g per kg) can be repeated, but ICP must be monitored.
2. Only short-term hyperosmolar therapy is useful because:
when serum sodium increases to >160 mEq per L, treatment is no longer useful,
dehydration can lead to cardiovascular collapse, and
a rebound increase in ICP occurs despite continued treatment, especially upon rehydration.
3. Patients given osmotic diuretics need bladder catheterization.
E. Hyperventilation and osmotic diuretics are used only if a definitive end point is first established, because beneficial effects are temporary. The target of hyperventilation is a pCO2 of 30 to 35 mm Hg (see Chapter 58).
F. Seizures.
1. Between 15% and 30% of patients with brain metastases have seizures.
3. Prophylactic antiepileptic drugs (AEDs) can be considered for patients with melanoma (50% of whom have seizures); otherwise there is no role for them. Posterior fossa metastases are not epileptogenic.
4. When phenytoin, dexamethasone, and whole-brain irradiation are used concurrently, the risk of erythema multiforme and erythema multiforme bullosa (Stevens-Johnson syndrome) increases.
5. Given the frequency of drug-drug interactions with older AEDs, newer agents should be considered first.
G. Surgical management.
1. The ideal candidate has minimal disability, a single, circumscribed, accessible lesion, and controlled systemic disease.
2. A shunt is indicated for obstructive hydrocephalus.
3. Current areas of controversy include:
Reoperation. A second resection may be considered for a lesion that recurs in the original tumor bed with minimal further parenchymal invasion.
Excision of multiple metastatic masses. If one or two of several metastatic lesions are symptomatic or life-threatening, palliative resection should be considered.
H. Radiation therapy (RT).
1. RT is the primary therapy for brain metastasis.
Standard dosage is 30 Gy to whole brain over 10 days; alternative dose-fractionation schedules are used in some cases.
Patients with radio-resistant tumors (melanoma and renal cell) may benefit from a radiosurgical boost to the tumor bed (section 3).
If dexamethasone is administered, acute complications of RT are few (mild headache, fatigue, hair loss, and asthenia).
CNS tolerance to RT is inversely proportional to the volume irradiated and dose used. Toxic effects include:
Acute encephalopathy: headache, nausea, and changes in mental status from increased ICP. Occurs within a few days and is common if steroids are not used during RT. Steroids in high doses help.
Early delayed encephalopathy: probably from demyelination; starts 14 to 120 days after RT with headache and drowsiness or brainstem dysfunction—ataxia, diplopia, and dysarthria. Spontaneous recovery in a few weeks is usual but steroids can help.
Delayed radiation encephalopathy: occurs months to years after RT. It manifests as diffuse cerebral atrophy, focal deficits, increased ICP, or as a normal pressure hydrocephalus (NPH) like syndrome (see Chapter 8). Pathology reveals necrosis—either from direct RT damage or vascular changes such as microangiopathy or accelerated atherosclerosis. A VP shunt is sometimes helpful.
Delayed radiation necrosis: usually occurs >1 year after RT; it can look and behave like a tumor.
Myelopathy: occurs within the first year of RT from demyelination and is usually transient.
Delayed severe myelopathy: occurs >1 year after RT from necrosis or atrophy, resembles cord compression with para- or quadriplegia but no pain. MRI is often normal. No specific treatment exists but steroids may help temporarily.
Plexopathy: RT-induced plexopathy can occur early but is usually delayed and must be differentiated from direct involvement of the plexus. Clues to RT damage include doses >60 Gy, painless weakness, lack of lymphedema or induration of the supraclavicular fossa, and presence of myokymic discharges on electromyography (EMG).
2. Prophylactic cranial irradiation is standard of care for limited stage small-cell lung cancer (SCLC); although it decreases the incidence of subsequent brain metastasis, it does not significantly affect patient survival.
3. Stereotactic radiosurgery (SR) or Gamma Knife delivers a single large or several smaller RT fractions to a well-defined, limited intracranial target with a sharp peripheral dose fall-off and minimal exposure to normal surrounding brain.
A 10 to 24 Gy fraction can be administered before or after conventional RT to tumor diameters of about 3 cm.
To remain within brain tolerance parameters, doses vary inversely with tumor size and the number of isocenters.
Local tumor control rates >80% can be achieved especially with radioresistant tumors, with complete response rates of approximately 40%.
Tumors <2 cm may respond better than larger ones and the risk of radiation necrosis is also less.
For patients with a single lesion, SR after whole-brain RT is beneficial. SR without whole-brain RT is controversial as local and distant brain control decreases but survival is unchanged.
4. A patient with radiation necrosis has acute or subacute neurologic deterioration and signs and symptoms of a mass lesion.
Neither CT nor MRI can help differentiate a necrotic mass from recurrent tumor. However, MR spectroscopy, MR perfusion, or PET imaging may.
Necrosis can be managed conservatively with steroids; recent data suggest using bevacizumab.
Resection is indicated if neurologic deterioration continues, escalating steroid doses become necessary, or intolerable steroid toxicity develops.
I. Chemotherapy.
1. As most tumors are drug-resistant, chemotherapy is not routinely indicated for management of CNS metastasis.
CNS metastases most frequently occur in patients with advanced, unsuccessfully treated cancers. Breast cancer patients who are Her-2 positive often have limited or no systemic disease but have CNS disease because trastuzumab cannot penetrate the CNS.
The blood-brain barrier may hinder penetration of most agents; hence, the CNS relapses.
2. Chemotherapy may be considered in
Patients with a chemosensitive tumor, good performance status, and inactive systemic disease with a metastasis that recurs after RT with or without surgery.
CNS metastasis from SCLC, breast, lymphoma, and germ cell tumors may respond to chemotherapy at rates comparable with those of the systemic tumor.
J. Cerebellar metastasis.
1. Common symptoms include: gait or limb ataxia, nystagmus, papilledema, headache, dizziness, vomiting, and double vision.
2. Initial treatments are the same as for supratentorial metastases: dexamethasone and RT.
3. Acute complications of RT are more common with cerebellar than with supratentorial metastases. Therefore, dexamethasone is started 48 hours prior to RT.
4. The risk of brain herniation after lumbar puncture (LP) is greater in patients with posterior fossa masses.
5. The indications for resection are the same as for supratentorial metastases, but any sign of clinical instability or deterioration, an expanding mass, hydrocephalus, or lack of response to dexamethasone should prompt consideration of immediate neurosurgical intervention except in brain stem lesions.
6. After resection of a cerebellar metastasis, patients have a higher incidence of developing leptomeningeal dissemination due to the proximity of the tumor to CSF spaces.
II. PITUITARY APOPLEXY
A. Pituitary apoplexy is an emergency. Acute panhypopituitarism occurs when a metastasis in the sella turcica or the pituitary gland necroses or hemorrhages—causing headache, ophthalmoplegia, bitemporal hemianopsia, blindness, encephalopathy, coma, hypotension and signs of meningeal irritation.
B. Treatment is with high IV doses of corticosteroids; surgical decompression may be needed.
C. Complete endocrine work up is indicated as hormone replacement will be needed.
III. METASTASIS TO THE SKULL BASE
The hallmark of metastasis to the skull base is involvement of the cranial nerves (CN). Most tumors arise from the breast, lung, or prostate. Five major syndromes have been recognized.
A. Orbital syndrome characterized by dull, continuous, progressive pain over the affected eye with proptosis, external ophthalmoplegia, and blurred vision. There is decreased sensation over the distribution of ophthalmic division of the trigeminal nerve (CN V).
B. Parasellar syndrome (cavernous sinus metastasis) manifests as unilateral frontal headache and ophthalmoplegia. Patients may have decreased sensation over the distribution of the ophthalmic division of CN. V. If cavernous sinus thrombosis occurs, there may be chemosis, edema of the eyelids and forehead, proptosis, and papilledema with retinal hemorrhages. In both the parasellar and orbital syndromes, steroids are warranted before RT to prevent acute vision loss from radiation-induced edema.
C. Middle fossa syndrome (Gasserian ganglion) is characterized by pain, numbness, or paresthesias over the distribution of the second (maxillary) or third (mandibular) divisions of CN V. The initial presentation may be a “numb chin” or a “numb lip.” Pterygoid and masseter weakness and abducens palsy occur late. About 65% of these lesions are from breast cancer and 15% from lymphomas. Approximately half the patients have mandibular metastasis, 15% have skull base lesions, and 20% carcinomatous meningitis.
D. Jugular foramen syndrome manifests as hoarseness and dysphagia (CN X) with or without pain (CN IX or X). Examination may show asymmetric palatal elevation (CN IX), weakness of the ipsilateral sternocleidomastoid and trapezius muscles (CN XI), and Horner’s syndrome (oculo-sympathetic). Weakness and atrophy of the tongue may be found if the tumor extends to the adjacent hypoglossal canal (CN XII).
E. Occipital condyle syndrome manifests as stiff neck and severe occipital pain that increases with neck flexion. Dysarthria and dysphagia from unilateral involvement of CN XII are seen in approximately 50%.
IV. DURAL METASTASIS
Dural metastases can cause headache or underlying venous sinus thrombosis and may invade the parenchyma. Malignant subdural effusions can also occur. Breast and prostate tumors are most commonly implicated.
V. SPINAL EPIDURAL METASTASIS
A. Metastatic epidural spinal cord compression (ESCC) occurs in approximately 5% to 10% of patients with cancer. ESCC is an emergency.
B. Prognosis. The most important determinant is neurologic function at presentation.
1. Ninety percent of ambulatory patients remain so after treatment, and have a 75% probability of surviving 1 year. Fewer than 10% of nonambulatory patients survive 1 year.
2. Only 50% of paraparetic and 13% of paraplegic patients with “radiosensitive” tumors become ambulatory after treatment.
3. Once neurologic dysfunction begins, paraplegia and loss of sphincter control occur within hours and are usually irreversible.
C. ESCC must be suspected on clinical grounds and must prompt timely confirmation and treatment.
1. About 60% of epidural metastates are from prostate, lung, breast, and kidney cancer; about 15% from multiple myeloma.
2. Approximately 50% of adults with an acute transverse myelopathy have metastatic ESCC. In 50% it is the initial manifestation of cancer, and in one half of those, the primary is lung cancer.
D. Presentation.
1. About 95% of patients with epidural tumor have progressive axial pain with or without a radicular or referred component.
2. Some weakness and sensory disturbance are present in 80% of patients.
3. Almost 60% of patients have sphincter dysfunction, a poor prognostic sign that implies bilateral cord or root damage.
E. Site of involvement.
1. The vertebral column, and never the intervertebral disks, is involved in about 85% of epidural tumors from solid cancers.
The vertebral body is involved in 45%.
The posterior arch and pedicle are involved in 40%.
The entire vertebra is involved in 15%.
2. About 50% to 70% of lesions involve the thoracic spine.
3. About 20% to 30% involve the lumbosacral spine.
4. About 10% to 20% involve the cervical spine.
5. At least one-third of patients with breast and prostate cancer have metastatic lesions at multiple levels.
F. There are three potential mechanisms of metastatic ESCC.
1. Most common is hematogenous spread to the vertebra.
2. The valveless veins in Batson’s plexus allow tumor seeding when the intraabdominal pressure increases.
3. Direct invasion of a paravertebral mass through the intervertebral foramen occurs in 75% of ESCC from lymphoma.
G. Diagnosis. Non-contrast-enhanced followed by contrast-enhanced MRI should be performed in all cases of suspected ESCC to establish the diagnosis and the extent of tumor invasion (Fig. 53.1). Because disease is often multifocal and discontinuous, the entire spine should be imaged. CT scan with or without myelography may be needed if an MRI is contraindicated.
H. Initial management.
1. If spinal cord compression is suspected, or upon confirmation, a 10-mg IV bolus of dexamethasone is administered and followed by 4 mg IV every 6 hours. If pain is severe, or if there is paresis or sphincter involvement, a 100-mg IV bolus of dexamethasone is administered and followed by 24 mg IV every 6 hours until more definitive treatment is started.
2. Maintenance dosage of dexamethasone (4 mg IV or orally every 6 hours) is then tapered as tolerated.
3. Steroids can promote clinical improvement and decrease pain but rarely produce a dramatic reversal of established neurologic disability. Prognosis is better when they do.
4. An indwelling bladder catheter should be inserted to check PVR and remain in.
5. Deep venous thrombosis prophylaxis and a stool-softening regimen should be started.
I. Although the intent of therapy remains palliative, several treatment options are available.
1. Surgery is indicated for establishing a diagnosis, in cases of spinal instability and/or presence of bone fragments within the spinal canal or continued neurologic decline during RT.
2. Decompressive laminectomy works only temporarily. It fails because
Metastatic tumor is generally located in the vertebral body (anterior to the spinal cord) and not in the neural arch.
Laminectomy may contribute to spinal instability.
3. Anterior vertebral body resection
This must be coupled with surgical stabilization of the spine.
Operative morbidity (10%) is from non-healing, breakdown, or infection of the wound and failure to stabilize the spine due to bone metastases.
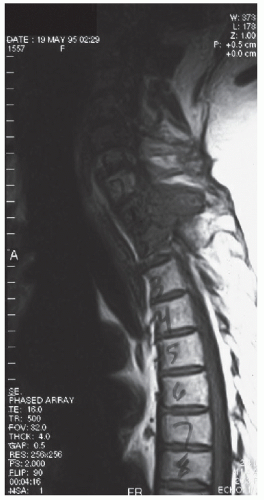 FIGURE 53.1 Sagittal MRI of the spine in a patient with metastatic ESCC. (Courtesy of José Biller, MD.) |
4. RT alone is the procedure of choice for radiosensitive tumors.
A total dose of 20 to 40 Gy divided over 10 to 20 fractions is the usual treatment—30 Gy is the most common dose.
The port should encompass two vertebral bodies above and below the epidural defect and any discontinuous lesions.
RT is indicated promptly after the diagnosis is made and should follow administration of dexamethasone.
If surgery is performed first, RT should follow after the wound heals.
The tumors that most commonly produce ESCC—lung, breast, prostate, and lymphoma—are likely to respond to RT.
If neurologic deterioration continues, surgical intervention should be considered.
The complications of RT are:
Myelosuppression.
Radiation myelopathy or syrinx (6 to 18 months after therapy).
Risk of a subacute syndrome characterized by Lhermitte’s sign several weeks after radiation.
5. Laminectomy plus radiation. For select patients, surgery followed by RT is better than RT alone for preserving function and increasing survival.
6. Epidural tumors do not respond rapidly enough to chemotherapy to warrant its use in most acute situations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








