10 Neuro-Ophthalmology Of the manifold symptoms which may be produced by an intracranial growth, none are of greater interest and none of greater importance than the various disturbances on the part of the visual apparatus. —Harvey Cushing, Johns Hopkins Hospital Bulletin, 1911 This chapter reviews the aspects of the ophthalmic, visual field, pupil, motility, and optic nerve exam most relevant to the neurosurgeon. Some essential aspects of neuro-ophthalmology and corneal preservation are summarized below: • By convention, confrontation fields are recorded from the patient’s perspective, so that results from the neurologic exam can be compared with formal perimetry. • Although bitemporal hemianopsia is the classic visual field defect in patients with chiasmal syndrome, many other field defects are possible. • The relative afferent pupillary defect (RAPD) should not be confused with the efferent pupillary defect (blown pupil) of CN III palsy due to aneurysms • When requesting an ophthalmology consult, always indicate whether the patient’s pupils can be dilated or not. • Papilledema should be excluded in all patients with cranial nerve (CN) VI palsy. • Patients with combined CN VII palsy and corneal anesthesia usually require a tarsorrhaphy. • A TegadermTM transparent dressing with lubricating ointment should be used rather than a cloth patch in patients with severe corneal exposure. • The visual acuity of each eye should be tested separately, ideally both near and at distance, with the appropriate refractive correction in place. Use the palm of the hand rather than fingers to occlude the eye because patients can see through the interdigital spaces. If glasses are not available, make a small pinhole through a piece of paper. Patients older than 40 years of age may require reading glasses or bifocals when testing the near vision. If patients are in the supine position, and the near vision is being tested, raise the glasses so that the bifocal segment at the bottom of the glasses will be in the proper position. • If patients are conscious but cannot verbalize, ask them to imitate fingers, or write down what they see. If patients do not appear conscious, document any aversion to bright light and the pupillary reaction to light. • Visual acuity can be obtained with an eye chart or near card. Normal visual acuity is at least 20/20, 6/6, or logMAR 0.00. The minimum visual acuity required for driving in most jurisdictions is 20/40, 6/12, or logMAR 0.30.1 Legal blindness can be defined as acuity in either eye less than 20/200, 6/60, or logMAR 1.00. If the patient cannot see the eye chart, then determine the maximum distance at which the examiner’s fingers can be accurately counted. If the patient is unable to count fingers, test if the patient can perceive when your hand starts and stops moving. If hand motions cannot be detected, then test for light perception with a bright light. Ensure that the contralateral eye is well occluded with a tissue and/or the palm of the hand. • The near and distance acuity measurements should correlate if the patient is using appropriate glasses. If the patient is unable to see the eye chart, but malingering is suspected, document the pupillary response and RAPD, and the optokinetic response. A positive optokinetic response usually indicates the vision potential is at least 20/400. • All one-eyed patients should be instructed to use dress polycarbonate (shatter-proof lens) glasses during waking hours. • Color vision loss is also known as dyschromatopsia. Color vision should be tested in each eye individually with the appropriate near correction. An asymmetric reading speed of the color plates may provide a clue to asymmetric optic nerve function. The most widely available test of color vision is the Ishihara color plates, but unfortunately 8% of the male population is red-green defective. Many color tests are available online and for smartphones. • Pupils should be tested in a dark room with the patient looking in the distance. Normal pupils should be round, equal in size, and constrict briskly to bright light. The size of the pupils in the light and the dark can be compared with a pupil gauge. If the pupillary reaction to light is normal, there is no need to test for the near reaction. Pupillary light-near dissociation occurs when the pupillary light reaction is poor, but the pupil reaction to near stimulus is better. An important cause of light-near dissociation in patients who present to the skull base surgeon is dorsal midbrain syndrome or Parinaud’s syndrome. • If the pupil is not round in the setting of facial trauma, gently elevate the lid to ensure that there is no globe rupture. An iris notch or tear in the iris sphincter with dilated pupil suggests traumatic mydriasis. Mimics of neurologically “blown” pupils include pharmacological dilation, angle closure glaucoma (misty cornea, mid-dilated pupil, elevated intraocular pressure, vomiting patient), sulcus intraocular lens after cataract surgery, and surgical iridectomy. • Horner’s syndrome is an oculosympathetic paresis, with ptosis, variable anhidrosis, and pupillary miosis (small pupil); it is more evident in the dark. The skull base surgeon may occasionally encounter Horner’s syndrome in the setting of brainstem lesions, cavernous sinus lesions usually with CN VI involvement, skull base osteomyelitis, neuroblastoma, or after Swan-Ganz catheter insertion (see also Chapter 7, Table 7.1). • Adie’s tonic pupil is a parasympathetic defect at the ciliary ganglion. Early on, the Adie’s pupil is mydriatic (enlarged pupil) and poorly reactive to light, with better reaction to a near stimulus. An isolated dilated pupil in a conscious patient with no extraocular muscle dysmotility or ptosis rarely, if ever, is associated with posterior communicating artery aneurysm. • When light is shone in one eye of a normal subject, both pupils will constrict equally. The pupillary reaction in the illuminated eye is the direct response, and the reaction in the contralateral eye is the consensual response. The afferent pupil fibers hemidecussate in the chiasm, as do the pupillomotor fibers in the brainstem. This double hemidecussation enables equal pupillary innervation, and equal pupil size. Examination Pearl When a bright light is shone in one eye, there should be an initial brisk constriction of the pupil, with equal contralateral pupillary constriction, due to the consensual response. • The swinging flashlight test compares the consensual pupillary light response of the two eyes. During the swinging flashlight test, if the pupil does not constrict, or frankly dilates when illuminated, an ipsilateral RAPD may be present. The RAPD is one of the most important objective signs in neuro-ophthalmology (Fig. 10.1). Fig. 10.1 Swinging flashlight test illustrating a left (OS) relative afferent pupillary defect (RAPD). Examination Pearl RAPD should not be confused with hippus, the rhythmic undulation in size of the pupil that is independent of illumination or accommodation, nor with the efferent pupillary defect (blown pupil) of CN III palsy due to aneurysm. • Test the confrontation fields in each eye separately. Static objects are usually more difficult for the patient to discern than moving ones (statokinetic dissociation). Therefore, asking patients to count fingers in each quadrant may be more accurate than moving in targets from the periphery and waiting for a “yes-no” response to perceived movement. • The Amsler grid tests the central 10 degrees of field and is especially useful for small homonymous occipital scotomas. • Whenever possible, correlate perimetric findings with the visual acuity and the fundus examination. Visual field defects that respect the horizontal midline are nerve fiber bundle defects, with the exception of lesions above or below the calcarine fissure. Visual field defects respecting the vertical midline are due to chiasmal or retrochiasmal pathology, or rarely retinal lesions on either side of the fovea. Most chiasmal field defects are bitemporal hemianopsias (Fig. 10.2a). It is uncommon for neurosurgical lesions to cause nasal field defects respecting the vertical midline.2 In the setting of binasal field loss, exclude optic nerve or retinal pathology, prior to considering intracranial lesions. • The more posterior the visual pathway lesion, the closer the corresponding visual fibers from the two eyes, and the more congruent the homonymous hemianopsia. Complete homonymous field defects do not precisely localize the retrochiasmal pathology. • Confrontation fields and Amsler grid testing are very useful, but formal perimetry can more accurately document and detect visual field loss. If patients are not cooperative for confrontation fields, they usually cannot perform formal perimetry either. • Tangent screens are no longer used by most ophthalmologists. Goldmann perimetry (manual or kinetic perimetry) is not as readily available as automated/computerized (static) perimetry. The most common automated perimetry test used for neuro-ophthalmic diagnosis is a central 24-degree or central 30-degree threshold test, with test points about 6 degrees apart. The only neurologic field defects that a central 30-degree test may miss are small occipital scotomas and the temporal crescent of an anterior occipital lesion. • In some countries the binocular Esterman field test is used as a screen to determine if the patient’s visual field can meet a minimum standard for driving. An international symposium suggested a minimum peripheral vision standard for driving of 120 degrees of uninterrupted horizontal peripheral vision, with at least 40 degrees of vertical peripheral vision.1 Fig. 10.2a,b (a) Bitemporal hemianopsia. (b) Junctional scotoma with lesion at the junction of the right optic nerve and chiasm. • Chiasmal lesions most commonly result in bitemporal hemianopsia. The bitemporal hemianopsia can be asymmetric, depending on the lateralization of the tumor. Monocular vision loss can result if a sellar mass compresses one optic nerve in a patient with a postfixed chiasm (See Fig. 3.2c, page 75). Junctional scotomas (monocular central scotoma with contralateral supero-temporal field loss, Fig. 10.2b) can occur when lesions compress the junction of the optic nerve and chiasm. Although the existence of Wilbrand’s knee has been questioned, junctional scotomas are a well-documented clinical finding. A pituitary mass can cause incongruous homonymous hemianopsia (optic tract syndrome) in a patient with a prefixed chiasm (see Chapter 3, Fig. 3.2b). • In general, pituitary tumors, growing from beneath the chiasm, cause bitemporal hemianopsia denser superiorly, whereas craniopharyngiomas compressing the chiasm from above cause bitemporal hemianopsia denser inferiorly, although this assumption is not always true. • After chiasmal decompression surgery, visual recovery may be rapid and often occurs within 24 hours. If preoperative optical coherence tomography (OCT, see below) of pituitary adenoma shows that the average retinal nerve fiber layer is less than 75 µm, the prognosis for vision recovery may be less favorable.3 • Pituitary apoplexy, when symptomatic, is characterized by headache, visual acuity loss (unilateral or bilateral), visual field loss, and dysmotility. CN III palsy is more common than CN IV or VI palsy.4 • Tumors of the medial sphenoid wing can compress the optic nerve and pre sent with early unilateral visual loss. Clinoidal meningiomas may also involve the cavernous sinus causing diplopia and facial numbness. Foster Kennedy syndrome (ipsilateral optic atrophy and contralateral papilledema) and painful ophthalmoplegia (Tolosa-Hunt syndrome) have been described with clinoidal meningioma (see also Chapter 7). • The normal cornea should be clear and glistening at all times. If there is any disruption of the corneal light reflex, a corneal epithelial defect should be suspected. A hand magnifier is a useful tool for bedside exams and can usually detect corneal foreign bodies, contact lenses, corneal abrasion, and Lisch nodules. • In patients with CN VII palsy, it is essential to check corneal sensation. The combination of a CN VII palsy and corneal anesthesia often necessitates tarsorrhaphy prior to discharge from the hospital. Corneal sensation can be assessed by tangentially touching the cornea in different quadrants with a sterile filament (e.g., 6-0 silk suture). Alternatively, artificial tears can be instilled in either eye, and the amount of subjective sensation compared. • If the direct ophthalmoscope is positioned about 50 cm from the eye, the red reflex can be examined. A central opacity in the red reflex may suggest a cataract. Posterior subcapsular cataracts in a young patient without history of diabetes mellitus or trauma may suggest neurofibromatosis type 2. • Digital palpation through the closed eyelids can sometimes suggest markedly elevated intraocular pressure, but usually this is an inaccurate estimate. To better assess the intraocular pressure, a Perkins tonometer or Tono-Pen can be borrowed from most emergency rooms, or from the ophthalmology operating room in the hospital. A vomiting patient complaining of a painful eye with a cloudy cornea and mid-dilated pupil suggests angle closure glaucoma. • In patients with corneal exposure, lubrication is essential. In ambulatory patients, lubricating drops can be used six times a day. If lubricating eyedrops are required more often than six times, preservative-free formulations can also be purchased over the counter. Lubricating ointment is a more effective moisturizer than drops, but it blurs the vision. For this reason many patients choose to use lubricating ointment only at bedtime. • In patients with prolonged inability to protect their cornea, suggest using maximal lubrication, eye glasses, and humidifier, and consider suggesting tarsorrhaphy, punctal occlusion, TegadermTM transparent dressing5/moisture chambers, Lacrisert®moisture pellets, botulinum ptosis, external lid load weight (stuck on with double-sided tape), or gold weight implantation. Eyelid weights work by gravity. Therefore, if weighting down the upper lid, the patient must sleep with the head elevated. • In a patient with poor eyelid closure, do not use a cloth eye patch. Cloth patches will abrade or desiccate the cornea. Instead, instill lubricating ointment and tape the eye closed or use a moisture chamber or intravenous plastic dressing (e.g., TegadermTM) over the affected eye. • Papilledema (nerve fiber layer edema) is bilateral optic disk edema from presumed increased intracranial pressure. Papilledema is caused by orthograde axoplasmic flow stasis at the optic nerve head. Patients with papilledema usually have retained central visual acuity, in contrast to the acuity loss in ischemic optic neuropathy and most cases of optic neuritis. The blood pressure should be checked in all patients with suspected papilledema, to exclude malignant hypertension. Usually diffuse fundus hemorrhages will accompany malignant hypertension, but these may be difficult to detect on direct ophthalmoscopy of patients with undilated pupils. Table 10.1 Modified Frisen Scale for Papilledema Grading6
 Introduction
Introduction
 Visual Acuity
Visual Acuity
 Color Vision
Color Vision
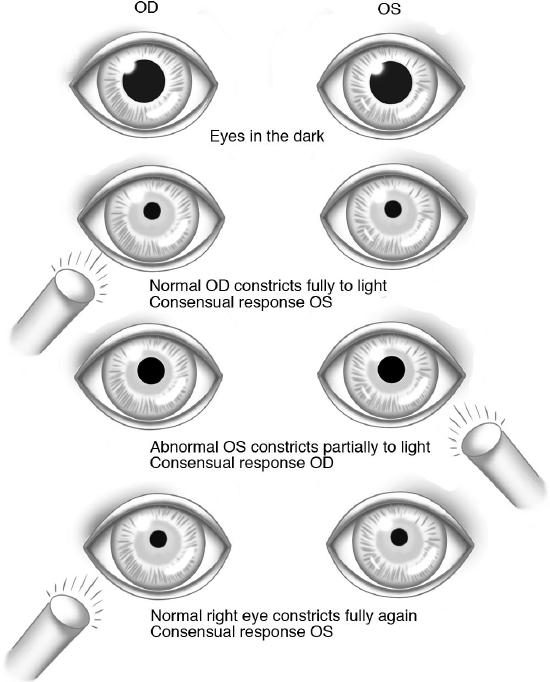
 Pupils
Pupils
 Although bilateral afferent pupillary defects can be seen, for example with bilateral optic neuritis, a RAPD cannot be bilateral. If a patient is suspected of having a relative afferent pupillary defect, there is often ipsilateral vision loss, visual field loss, and color vision loss. However, there are some important exceptions to consider. A midbrain lesion at the brachium of the superior colliculus can cause RAPD without acuity or field loss, because the geniculocalcarine fibers are not involved. Patients with unilateral optic neuritis may often have an ipsilateral RAPD, but retain 20/20 acuity. There may be decreased color perception, loss of contrast sensitivity, motion sensitivity, or visual field loss. Vision loss does not always correlate with an RAPD. A macular hole at the fovea of the retina may greatly decrease acuity, but because the amount of retina involved is small, there is no RAPD. A dense unilateral cataract may cause blindness, but should not cause an ipsilateral RAPD.
Although bilateral afferent pupillary defects can be seen, for example with bilateral optic neuritis, a RAPD cannot be bilateral. If a patient is suspected of having a relative afferent pupillary defect, there is often ipsilateral vision loss, visual field loss, and color vision loss. However, there are some important exceptions to consider. A midbrain lesion at the brachium of the superior colliculus can cause RAPD without acuity or field loss, because the geniculocalcarine fibers are not involved. Patients with unilateral optic neuritis may often have an ipsilateral RAPD, but retain 20/20 acuity. There may be decreased color perception, loss of contrast sensitivity, motion sensitivity, or visual field loss. Vision loss does not always correlate with an RAPD. A macular hole at the fovea of the retina may greatly decrease acuity, but because the amount of retina involved is small, there is no RAPD. A dense unilateral cataract may cause blindness, but should not cause an ipsilateral RAPD.
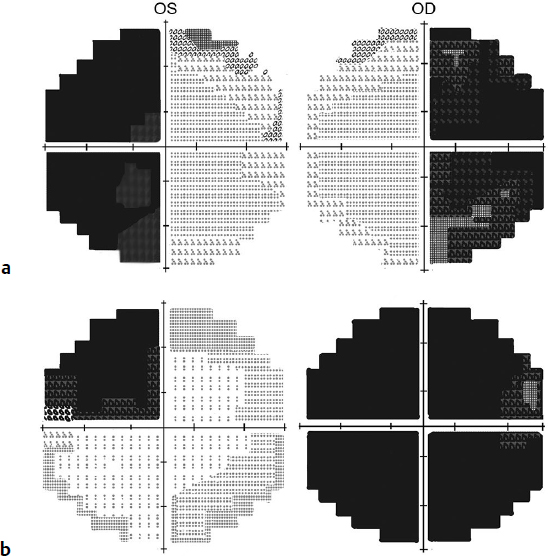
 Visual Fields
Visual Fields
 Pituitary Tumors, Chiasmal Syndromes, and Medial Sphenoid Wing Meningioma
Pituitary Tumors, Chiasmal Syndromes, and Medial Sphenoid Wing Meningioma
 Anterior Segment Examination
Anterior Segment Examination
 Corneal Exposure
Corneal Exposure
 Fundus Examination
Fundus Examination
 A modified Frisen scale can be used to clinically grade papilledema6 (Table 10.1).
A modified Frisen scale can be used to clinically grade papilledema6 (Table 10.1).
 Optical coherence tomography (OCT) is akin to an optical ultrasound, and provides a more objective measure of the nerve fiber layer height, and the progression of papilledema. In patients with papilledema and decreased retinal nerve fiber layer depth on OCT, it may be difficult to distinguish resolution of papilledema from atrophy of the nerve fiber layer. OCT analysis of the retinal nerve fiber layer and retinal ganglion cell layer in papilledema can be associated with misleading artifacts due to layer segmentation failures.7
Optical coherence tomography (OCT) is akin to an optical ultrasound, and provides a more objective measure of the nerve fiber layer height, and the progression of papilledema. In patients with papilledema and decreased retinal nerve fiber layer depth on OCT, it may be difficult to distinguish resolution of papilledema from atrophy of the nerve fiber layer. OCT analysis of the retinal nerve fiber layer and retinal ganglion cell layer in papilledema can be associated with misleading artifacts due to layer segmentation failures.7
Grade | Definition/Findings |
0 | Normal optic disk |
I | Minimal papilledema: subtle C-shaped halo of disk edema with a normal temporal disk margin |
II | Low-degree papilledema: circumferential halo of disk edema |
III | Moderate papilledema: obscuration of one or more segments of the major blood vessels leaving the disk |
IV | Marked papilledema: partial obscuration of a segment of major blood vessel on the disk |
V | Severe papilledema: partial or total obscuration of all blood vessels on the disk |
Note: for further information, go to http://content.lib.utah.edu/utils/getfile/collection/EHSL-Moran-Neuro-opth/id/140/filename/88.pdf.
 Despite OCT, distinguishing papilledema from pseudopapilledema may be difficult unless surface disk drusen or peripapillary nerve fibers can be identified. Further advances in OCT may enable axonal diameters to be accurately measured, thus facilitating a more definitive determination of subtle papilledema.
Despite OCT, distinguishing papilledema from pseudopapilledema may be difficult unless surface disk drusen or peripapillary nerve fibers can be identified. Further advances in OCT may enable axonal diameters to be accurately measured, thus facilitating a more definitive determination of subtle papilledema.
 OCT testing is most helpful when the measurements are normal. Structural measurements of the optic nerve in and of themselves may not be adequate for diagnosis. OCT measurements should be interpreted in light of severity and duration of vision loss, eye findings, perimetry, magnetic resonance imaging (MRI) scan, and prior OCT if available.
OCT testing is most helpful when the measurements are normal. Structural measurements of the optic nerve in and of themselves may not be adequate for diagnosis. OCT measurements should be interpreted in light of severity and duration of vision loss, eye findings, perimetry, magnetic resonance imaging (MRI) scan, and prior OCT if available.
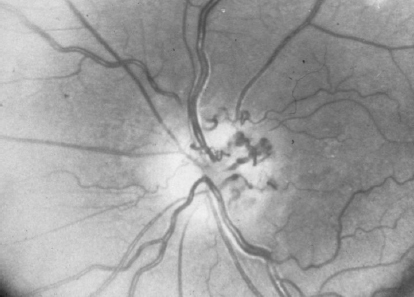
• Peripapillary retinochoroidal collaterals (often inappropriately called “optociliary shunt”) may be seen with optic nerve sheath meningioma, optic nerve glioma, and chronic papilledema (Fig. 10.3). However, retinochoroidal collaterals are not specific for optic nerve tumors and are more commonly seen with central retinal vein occlusion.
• Nonarteritic ischemic optic neuropathy is a common age-related optic neuropathy, attributed to an ischemic spiral in an optic nerve that usually has a small cup/disk ratio. Bilateral sequential non-arteritic ischemic optic neuropathy more frequently causes the appearance of a unilateral swollen disk with contralateral disk pallor (Fig. 10.4), than does Foster Kennedy syndrome (e.g., olfactory groove meningioma with ipsilateral optic atrophy and contra-lateral papilledema).