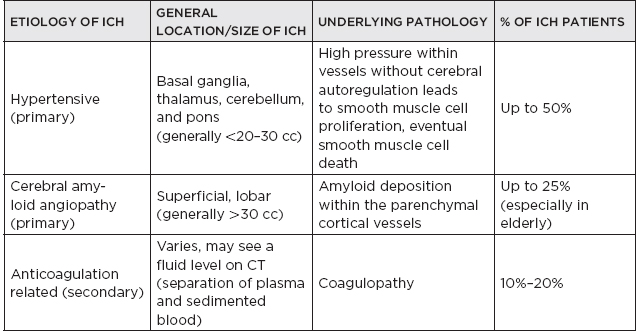
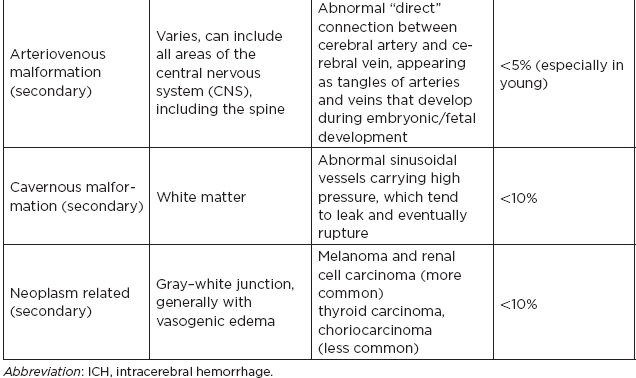
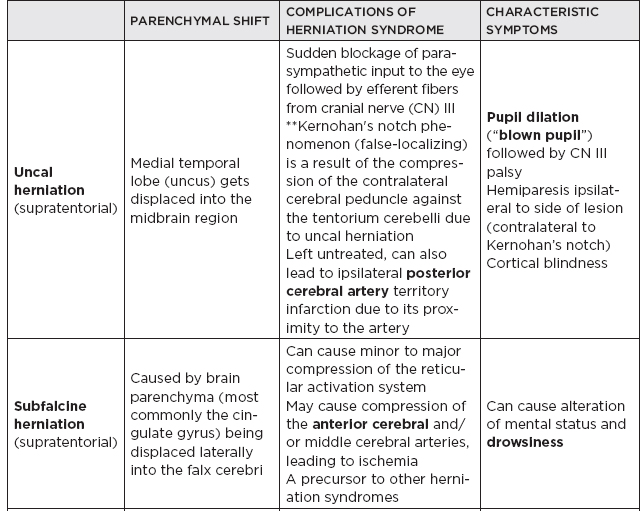
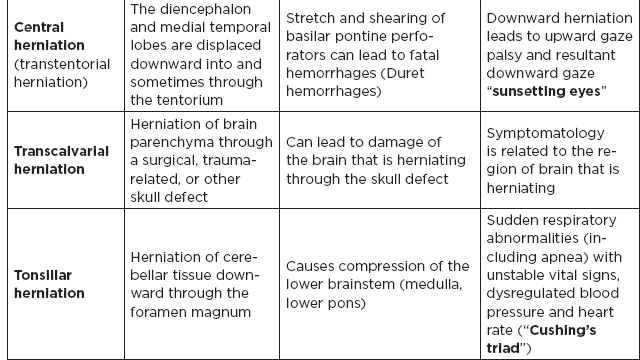
CHAPTER 7 Neurocritical Care I. Critical Care Management of Acute Ischemic Stroke A. Acute ischemic stroke management is discussed more extensively in Chapter 5. Majority of stroke patients are managed on a regular or step-down nursing floor. Approximately 15% to 20% of stroke patients are admitted to an intensive care unit, mainly for the following indications: • Monitoring and treatment of malignant cerebral cytotoxic edema (“hemicraniectomy watch”) • Monitoring hemorrhagic transformation of an acute ischemic stroke • Airway management due to neurological deficits of stroke • Intractable seizure management • Aggressive blood pressure (BP) augmentation/control • Neurological monitoring post intra-arterial intervention or intravenous tissue plasminogen activator (tPA) 1. Malignant cerebral edema management (“hemicraniectomy watch”) a. The definitive treatment of malignant cerebral edema due to large territory stroke is hemicraniectomy or suboccipital decompression, generally within 48 hours of infarction. i. Involves the surgical removal of the skull proximal to the affected area of brain for resolution of pressure and compression due to cerebral edema ii. Reduces mortality, not necessarily morbidity iii. Better functional outcomes in patients younger than 60 years of age b. Hyperosmolar therapy (medical management)—used to “shrink” normal (unaffected) brain by drawing water out of living brain cells into the vasculature i. Hypertonic saline (A) 2% hypertonic saline—can be given by peripheral access (B) 3% hypertonic saline—central access only (vesicant) (C) 23.4% hypertonic saline “bullet”—central access only (vesicant) (1) Given for neurological emergencies (e.g., herniation) ii. Mannitol push (0.5 g–1 g/kg)—can be given by peripheral access c. Other adjunct therapies to reduce intracranial pressure that may be considered i. Therapeutic hypothermia (systemic cooling generally from 32–36°C) ii. Elevating head of the bed iii. Hyperventilation (prolonged hyperventilation can cause further ischemia) iv. Anesthetics (propofol, pentobarbital) v. Extraventricular device placement (in theory can lead to upward herniation) 2. Hemorrhagic transformation management a. Hemorrhage after ischemic stroke (hemorrhagic transformation) is common (20%–40%). b. It is more common in patients with microbleeds seen on gradient echo MRI, in patients treated with tPA, and in older patients. c. There is a higher predilection for hemorrhagic transformation if the patient has: i. Hypertension (>185/110 mmHg) ii. Hyperglycemia (especially >200 mg/dL) iii. Anticoagulation iv. A National Institute of Health Stroke Scale (NIHSS) score of greater than 20 v. A larger stroke size (>1/3 territory) vi. Early venous filling discovered on angiography d. The current intensive care unit (ICU) management of hemorrhagic transformation is risk factor management of: i. Refractory hypertension: Aim to keep mean arterial pressure (MAP) less than 130, diastolic BP (DBP) less than 105, systolic BP (SBP) less than 220 mmHg. (A) Prehospitalization BP (baseline SBP/DBP) should be taken into consideration when aggressively lowering BP so as to not cause further ischemia. (B) Utilization of intravenous calcium channel blockers such as nicardipine or hydralazine or a mixed alpha and beta antagonist such as labetalol is recommended. (C) Nitroprusside, although effective at decreasing blood pressure rapidly, is uncommonly used because it leads to venodilation, which may lead to increased intracranial pressure (ICP). ii. Hyperglycemia: can be managed with long-acting insulins, short-acting insulins, and insulin drips. American Heart Association/American Stroke Association (AHA/ASA) recommends frequent monitoring of glucose, in all acute stroke patients, especially in known diabetics, with a goal of between 80 and 200 mg/dL and a strict goal to avoid hypoglycemia. Stroke patients with higher admission glucose levels have been shown to have poorer neurological outcomes. However, acute aggressive reduction has not been shown to be beneficial. iii. Anticoagulation: heparin drip, if needed due to high-risk conditions (e.g., mechanical valve prophylaxis, left ventricular thrombus) with close neurological monitoring. 3. Seizure management of acute stroke patients a. First-time seizures occur in less than 5% of stroke patients. b. Seizures in stroke patients are generally an indicator of a larger infarction, generally involving the cortex. Patients with larger strokes, cortical involvement, and hemorrhage are at higher risk for post-stroke seizures. c. There are a number of abnormal movements that can result from stroke that are not related to seizures, and it is important to differentiate seizures from nonepileptiform movement disorders. d. Antiepileptic drugs/medications (AEDs) i. AEDs should not be prescribed in the post-stroke setting if the patient does not have a seizure disorder (i.e., prophylactically) because they have been associated with worsened outcomes (e.g., phenytoin), increased sedation, cognitive impairment, and improper duration of therapy. ii. AEDs should be prescribed for at least a short term (3–6 months) in the post-stroke setting if the patient has a seizure within the first weeks after an acute ischemic stroke. Reassessment of the need for AEDs should be made in follow-up visits because a significant number of patients recover from their need for AED therapy. iii. Continuous EEG monitoring (cEEG) should be considered in post-stroke patients with impaired consciousness or altered mental status to assess for nonconvulsive seizure activity iv. After hemicraniectomy, AEDs can be considered for a short term (generally for up to a week) because there is a higher predilection to seizures in the immediate postoperative period. 4. Blood pressure augmentation/reduction and cardiac considerations: arterial line placement is ideal in the titration of vasoactive medications. a. Augmentation: patients may have symptomatic penumbral oligemia surrounding the core infarct (“perfusion-dependence”) that can be theoretically salvaged by induced hypertension. Mean arterial pressures (MAPs) are generally augmented by normal saline boluses or vasopressive medications (e.g., phenylephrine, norepinephrine). b. Reduction: most patients should have a goal of MAP less than 130, DBP less than 105, and SBP less than 220 mmHg without aggressive reduction (no more than 10% reduction per hour). Utilization of calcium channel blockers (hydralazine, nicardipine) and/or alpha-beta blockade (labetalol) may be of use in the acute setting. c. Other cardiac considerations: Cardiopulmonary effects of acute ischemic stroke include neurogenic pulmonary edema (catecholamine release mediated), stunned myocardium, and myocardial infarction. Echocardiogram, electrocardiogram, and cardiac enzymes should be assessed on most if not all stroke patients to monitor for cardiac changes. 5. Neurological monitoring after tPA and/or intra-arterial therapy: during and immediately following tPA infusion, there is a heightened predilection for patients to have intracerebral and other bleeding diathesis. A number of assessments are required, including: a. Neurological assessments and vital sign checks every 15 min during tPA infusion b. Continued neurological monitoring every 15 min after infusion for the first hour c. Continued neurological monitoring every 30 min after infusion for the next 6 hours d. Hourly neurological assessments after infusion for the remaining hours until 24-hour CT scan is obtained e. Continuous monitoring for angioedema or other reactions, and minor or major bleeding, for first 24 hours after tPA infusion II. Critical Care Management of Acute Intracerebral Hemorrhage A. General principles of intracerebral hemorrhage (ICH) 1. ICH constitutes 15% to 20% of new strokes annually, with an incidence of 15 per 100,000 people. 2. Functional morbidity is seen in up to 80% of patients, and up to 50% of patients suffer mortality within 30 days. a. It is unknown if much of the morbidity and mortality is due to the primary effect of the ICH or the secondary inflammatory responses to blood degradation products, microglial infiltration, and cerebral edema. b. Hematoma volume at presentation is currently the best predictor of clinical outcome. 3. Longstanding hypertension is the most common cause of ICH (about 50%). a. Hypertensive ICH is thought to arise mainly from lenticulostriate arteries, which stem from the major cerebral vessels but, unlike the larger vessels, do not benefit from cerebral autoregulation of blood pressure. Pathological changes can include liophyalinosis of small arterioles and microaneurysms of perforating arteries (Charcot-Bouchard aneurysms). b. Hypertensive ICH occurs mainly in deep brain structures such as the basal ganglia (especially putamen), thalamus, pons, and cerebellum. 4. Anticoagulants, including the new oral anticoagulants (NOACs), and pathological processes such as cerebral amyloid angiopathy (lobar hemorrhage, often recurrent) and blood vessel malformations account for most of the remaining ICH etiologies. Statin use has also recently been implicated as a potential risk factor in the development of ICH. Common Etiologies of Primary and Secondary Intracerebral Hemorrhage B. ICH expansion 1. Nearly half of all ICHs will expand in size from initial presentation within the first few hours, and most if not all of these patients should be monitored in an appropriate ICU. 2. Hematoma expansion generally stabilizes within 24 hours and is more often observed in hypertensive patients and in patients with poorly controlled diabetes. 3. Antiplatelet use has not been shown to the increase risk for ICH expansion, and evidence for platelet transfusion in patients who have been using antiplatelet medications is currently lacking. 4. CT angiogram in the acute setting may be helpful in identifying a bleeding source (“spot sign”), which, if found, is a relatively strong predictor of hematoma expansion. C. Supportive treatment of ICH 1. Intubation for rapidly declining neurological status or a Glasgow Coma Scale (GCS) score of less than 8 a. For large hemorrhages, controlled hyperventilation to a partial pressure of carbon dioxide (PCO2) of 25 to 30 mmHg for short periods of time may be beneficial. 2. Maintenance of euvolemia 3. Close glucose monitoring and electrolyte normalization 4. Prevention of deep venous thrombosis (DVT) with subcutaneous heparinoids (generally started 24–48 hours following ICH ictus) and compression stockings 5. Management of cardiopulmonary effects of ICH, including neurogenic pulmonary edema, troponin leak due to myocardial infarction, and stunned myocardium 6. Treatment of seizure disorder secondary to ICH 7. ICP monitoring (especially in patients with GCS <8) with intra-parenchymal or external ventricular device placement for patients with hydrocephalus and declining mental status; goal of ICP less than 20 mmHg and cerebral perfusion pressure (CPP) less than 50 mmHg 8. The use of tissue-dehydrating agents: mannitol (with osmolality kept between 295 and 305 mOsm/L and Na at 145–150 mEq), or hypertonic saline (generally 2% or 3%) for tissue swelling or impending herniation 9. Management of ICH-related fever D. Adjunctive treatments for ICH: in addition to the supportive treatment for ICH, there is increasing evidence that adjunctive therapies for ICH may be beneficial for patients with certain conditions. 1. Anti-epileptic medications/drugs a. Clinical seizures are common after ICH (10%–30%). b. Continuous EEG monitoring may be beneficial in patients with fluctuating or depressed neurological exam. c. AEDs are controversially used for seizure prophylaxis as clinical studies have shown limited benefit and some observational studies have shown worsened outcomes with phenytoin. d. AEDs should only be prescribed for clinical or electrographic seizures due to ICH. The duration of AED therapy should be readdressed after the acute phase of ICH. 2. Anticoagulant reversal a. Vitamin K antagonist: warfarin i. Fresh frozen plasma and intravenous vitamin K are the current mainstay of therapy for warfarin-related ICH because they are effective in normalizing the prothrombin time/international normalized ratio (PT/INR). Prothrombin complex concentrates (PCCs) are becoming increasingly common because they have stronger therapeutic efficacy and faster effect; however, they are costly, and no difference in clinical outcomes have been proven. b. Factor Xa inhibitors: rivaroxaban, apixaban, edoxaban i. Andexanet alfa (rivaroxaban, apixaban, edoxaban antidote) is a therapeutic option that is currently being studied for the more immediate reversal of the Xa inhibitor NOACs. Dialysis, fresh frozen plasma, 4-factor prothrombin complex, anti-inhibitor coagulant complex (FEIBA), and cryoprecipitate have also been hypothesized to be beneficial in symptomatic hemorrhages. c. Direct thrombin inhibitor:dabigatran i. Idarucizumab (dabigatran antidote) has been shown in a recent study to reverse the activity of dabigatran and underwent fast-track approval by the U.S. Food and Drug Administration (FDA) for clinical use. d. Antithrombin III activator/Xa inhibitor: heparin, enoxaparin i. Protamine sulfate dosed at 1.0 to 1.5 mg per 100 IU of heparin (or per 1 mg of enoxaparin) if it can be dosed within the first 30 minutes since the cessation of heparin to a maximum of 50 mg protamine sulfate ii. Protamine sulfate dosed at 0.5 to 0.75 mg per 100 IU of heparin (or per 1 mg of enoxaparin) if it can be dosed from 30 minutes to 1 hour after heparin cessation 3. Aggressive blood pressure control a. Initially a more debated topic, aggressive BP control was considered the mainstay of ICH therapy because it was thought to limit hematoma expansion and end-organ damage. b. Recently some evidence suggested that aggressive BP control may lead to increased hematoma expansion due to bleeding into peri-hematomal cerebral ischemia. c. Recent studies show that patients radiologically appear better with more aggressive BP lowering (SBP <140); however, further studies are ongoing to evaluate clinical outcomes. 4. Surgical management of ICH a. The surgical trial in ICH (STICH) failed to show benefit of surgical hematoma evacuation for supratentorial bleeds. There were some analyses showing that superficial cortical hemorrhages may benefit from surgery more than deeper ICHs. b. Catheter-based studies infusing tPA into the hemorrhage with clot aspiration have shown some outcome benefit. c. Surgical management is recommended for infratentorial ICH if larger than 3 cm to reduce morbidity and mortality. d. Patients suffering ICH with intraventricular component may benefit from tPA instillation via intraventricular catheter, although data for this are still being analyzed. e. The efficacy of endoscopic (minimally invasive) resection of blood products from ICH currently is being studied. f. Iron chelation from ICH-related blood currently is being studied. E. ICH scoring ELEMENT SCORING GCS 13–15 (0); 5–12 (1); 3–4 (2) Age <80 (0) ; > or = 80 (1) Infratentorial No (0) ; Yes (1) Volume <30 cc (0); >30 cc (1) Intraventricular Blood No (0) ; Yes (1) Mortality by Score 1 (13%), 2 (26%), 3 (72%), 4 (97%), 5 (>99%), 6 (>99%) F. Diagnosing ICH etiology 1. ICH and ICH stability are easily diagnosed on plain head CT. 2. CT angiogram can be used to determine if there is a “spot sign” (active extravasation), which is a strong predictor of hematoma expansion. 3. All patients with ICH under the age of 40 and/or without evidence of hypertension should undergo diagnostic digital subtraction angiography for detection of vessel abnormality (arteriovenous malformation [AVM], dural venous fistula, etc.). 4. MRI should be completed on most patients with ICH to further clarify etiology utilizing gradient echo (GRE), T1 and T2 sequences. a. Cavernomas have a characteristic “popcorn” appearance on T2 sequences (low intensity on T2WI and GRE [hemosiderin] surrounding various circumscribed regions of hemorrhage [hyperintense on T1WI because of the presence of methemoglobin]) b. Cerebral amyloid angiopathy shows up with numerous microbleeds as “Swiss-cheese brain” on gradient-echo or susceptibility weighted imaging. c. Tumors generally show up as contrast-enhancing lesions on post-contrast T1 imaging. d. Blood aging can also be better determined by MRI sequences: T1 T2 Hyperacute 0–24 hours (oxyhemoglobin) Isointense Hyperintense Acute 1–3 days (deoxyhemoglobin) Isointense Hypointense (dark) Early subacute 3–7 days (methemoglobin) Hyperintense (bright) Hypointense (dark) Late subacute 7–30 days (extracellular methemoglobin) Hyperintense (bright) Hyperintense (bright) Chronic >30 days (hemosiderin) Hypointense (dark) Hypointense (dark) III. Critical Care Management of Subarachnoid Hemorrhage and Delayed Cerebral Ischemia A. General principles of unruptured intracranial aneurysms and subarachnoid hemorrhage: 1. Subarachnoid hemorrhage (SAH) is a neurological disorder that is caused by the rupture of a cerebral artery, causing blood to collect in the subarachnoid space. 2. Trauma is the most common cause of SAH. 3. Up to 80% of nontraumatic SAH is caused by intracranial saccular aneurysm rupture, making it the most common cause of nontraumatic SAH. 4. About 2% of the general population harbors an unruptured intracranial aneurysm, and aneurysmal SAH occurs at an estimated rate of 6 to 16 per 100,000 population (30,000 people in the United States suffer an SAH annually). 5. Screening is recommended for family members with two or more first-degree relatives with history of SAH or brain aneurysm. 6. Common sites of aneurysm include: a. Anterior communicating artery (30%–40%), b. Internal carotid artery (30% including all cavernous and supraclinoid segment aneurysms), c. Middle cerebral artery (20%–30%) d. Vertebrobasilar arteries (10%). 7. Aneurysms occur more frequently in females (3:1) 8. Other risk factors associated with aneurysm formation include: a. Cigarette smoking b. Hypertension c. Age d. Inherited genetic conditions i. Autosomal dominant polycystic kidney disease ii. Vascular type Ehlers-Danlos syndrome iii. Hereditary hemorrhagic telangiectasia iv. Pseudoxanthoma elasticum 9. Aneurysm rupture risk is related to size, with larger aneurysms predicting a higher risk of rupture. Posterior circulation and posterior communicating aneurysms potentially are associated with a higher risk of rupture compared to anterior circulation aneurysms. 10. There is a high mortality rate (>30%) in the field on initial bleed. Of the patients who survive the ictal bleed, about one-third die in the hospital, one-third live with significant disability, and one-third recover with little disability. 11. One of the major complications that arises generally between 4 and 12 days post-SAH is delayed cerebral ischemia (DCI), which is the development of a secondary stroke or neurological deficit. DCI is the cause of nearly half of the disability and death in patients hospitalized for SAH. a. DCI has been rarely reported earlier than 3 days and later than 21 days post-SAH. b. Transcranial Doppler has been a standard monitoring modality to assess cerebral artery vasospasm, which is commonly thought to be associated with and potentially causative of DCI. c. It is important to note, however, that DCI and cerebral artery vasospasm are independent entities that occur after SAH, and one may happen without the other. d. Aneurysmal rupture is commonly associated with DCI, whereas traumatic SAH is less often associated with complications of DCI. B. Grading of subarachnoid hemorrhage GRADE HUNT AND HESS CLASSIFICATION Grade I Asymptomatic or with slight headache Grade II Moderate to severe headache, nuchal rigidity, no focal or lateralizing signs Grade III Drowsy, confusion, mild focal deficit Grade IV Persistent stupor, semicoma, early decerebrate rigidity Grade V Deep coma and decerebrate rigidity GRADE MODIFIED FISHER SCALE Grade 0 No subarachnoid or intraventricular blood noted on CT scan Grade I Thin subarachnoid blood with no intraventricular component Grade II Thin subarachnoid blood with intraventricular component Grade III Thick subarachnoid blood with no intraventricular component Grade IV Thick subarachnoid blood with intraventricular component C. Management of cerebral arterial vasospasm: • Post-SAH vasospasm is specifically defined as symptomatic or asymptomatic delayed narrowing of the large cerebral arteries that is associated with clinical or radiographic signs of ischemia following a SAH. • It is commonly found in patients generally 3 to 7 days following SAH, and the gold standard imaging modality for workup is the digital subtraction (catheter) angiography. However, monitoring is also done noninvasively (transcranial Doppler [TCD], CT angiography [CTA]/CT perfusion [CTP], electroencephalogram, etc.). 1. TCD monitoring: very common, noninvasive technique to assess for vasospasm. Very specific but variable sensitivity for vasospasm and is proceduralist dependent. Important numbers indicating possible vasospasm: a. Velocity >200 cm/s (severe in middle cerebral artery [MCA]) b. Rapid rise between serial TCD measurements (>50 cm/s) c. High ratio of MCA velocity to ipsilateral extracranial internal carotid artery (ICA) (Lindegaard Index) d. Cannot complete if thickened temporal bones e. Like other ultrasound studies, visualization is highly operator dependent. 2. CTA/CTP: Is moderately sensitive (>70%), very specific (>90%) a. Requires iodinated dye load and CT radiation b. Overcomes anatomical limitations of TCD 3. Catheter angiogram a. Gold standard, most invasive, requires iodinated dye load b. Confirm diagnosis of vasospasm c. Can offer endovascular therapy (intra-arterial [IA] vasodilators such as verapamil, nicardipine, or balloon angioplasty) for treatment of vasospasm 4. Emerging adjunct (“multi-modality”) monitoring for DCI in SAH patients: includes electroencephalogram, brain tissue oximetry, microdialysis, near-infrared spectroscopy, thermal diffusion flowmetry D. Routine screening and management of high risk DCI in SAH patients in the critical care unit 1. There are several clinical signs and symptoms that can lead a clinician to suspect DCI: a. The presence of a new neurological deficit on clinical exam b. A sudden increase in BP c. An increasing level of velocities on TCD (or increased ratio) d. New strokes on imaging 2. The following are some techniques that can help in the screening of DCI in the SAH patient: a. TCD every day for at least 14 days b. Frequent neurological checks (every 2 hours to every 4 hours) c. Consideration of multi-modality monitoring (see Section III.C.4) 3. “Triple-H” therapy: Many neurological ICUs employ the use of triple-H therapy in the treatment and prophylaxis of DCI. There is relatively weak evidence for this particular therapeutic approach, however. After the aneurysm is secured, the main goal is to maintain cerebral perfusion. Triple-H therapy employs the following: a. “Hemodilution”—theoretically improves rheology to increase brain tissue oxygen perfusion. However, studies have not shown that a lower hemoglobin (Hgb) is beneficial. Anemia is also associated with poorer outcomes in SAH patients. A target Hgb of 8 g/dl to 10 g/dl is considered by expert opinion to be most beneficial; however, increased phlebotomy to drop hemoglobin is not advised. b. “Hypertension”–permissive/induced (unless heart failure, myocardial infarct, etc.): in patients with DCI, can trial induced hypertension to a MAP 10% above baseline with fluid boluses, or to symptom resolution. If there is improvement, can consider norepinephrine or phenylephrine or other medication for the hemodynamic augmentation of BP. c. “Hypervolemia” (volume expansion)—goal is ideally for euvolemia with the avoidance of intravascular volume depletion. Hypervolemia has not shown improved outcomes. Fluid balance should be closely monitored, and routine placement of pulmonary artery catheters or other invasive methods should be avoided unless the balance is difficult to ascertain. 4. Angioplasty/IA therapy: patients who are refractory to or are unable to tolerate the previously discussed medical therapies should be considered for endovascular spasmolytic therapies. These therapies (usually angioplasty or IA calcium channel blocker) should be directed only at vessels that are thought to be causing clinical deficit. 5. Nimodipine in the SAH patient: nimodipine was initially used for its calcium-channel-blocking properties because the mechanism of vasospasm was thought to begin with cerebral smooth muscle contraction. SAH patients, however, had improved outcomes with nimodipine, but not with other calcium channel blockers, and patients receiving oral nimodipine continued to show angiographic vasospasm. The improved outcomes related to oral nimodipine are now thought to be possibly secondary to protective effects on the neurovascular unit. Additionally, due to its continued benefit seen in multiple large randomized studies, oral or nasogastric nimodipine is strongly recommended (level I-A) in post-aneurysmal SAH patients for 21 days. If the patient’s BP does not tolerate a dose of 60 mg of nimodipine every 4 hours (i.e., not able to meet physiological or physician set MAP goals), a dose of 30 mg every 2 hours may be trialed. IV. Other Intracranial Vascular Malformations A. Arteriovenous malformations 1. Consists of multiple arteries and veins, connecting at a nidus without an intervening normal capillary bed. Pathogenesis is not well understood, but they are considered sporadic congenital developmental vascular lesions 2. Brain AVMs occur in about 0.1% of the population (one-tenth the incidence of intracranial aneurysms) Supratentorial lesions account for 90% of brain AVMs, and the remainder are in the posterior fossa. They usually occur as single lesions, but as many as 9% are multiple. 3. Brain AVMs usually present between the ages of 10 and 40 years, with ICH, seizure, headache, or focal neurologic deficits. Hemorrhage is the most common presentation, particularly in children. 4. Overall, annual hemorrhage rates from brain AVMs are between 2% and 3%. After an initial hemorrhage, annual hemorrhage rates are approximately 6% to 17% in the first year, but then decrease. 5. Diagnosis can be made by CT or MRI. MRI is more sensitive, particularly in the setting of an acute ICH. Digital subtraction angiography (DSA) is the gold standard for the diagnosis, treatment planning, and follow-up after treatment of brain AVMs 6. Important considerations in the decision to treat and the choice of treatment are age, lesion size and location, and prior history of intracerebral hemorrhage. 7. Surgery is the mainstay of treatment; radiosurgery is a useful option in lesions deemed at high risk for surgical therapy, and endovascular embolization can be a useful adjunct to these techniques. B. Cavernous malformations 1. Cavernomas are thin-walled dilated capillaries with a simple endothelial lining. 2. May occur as a sporadic or familial condition 3. Three genetic loci (CCM1, CCM2, and CCM3) responsible for familial cavernous malformations have been reported. Nearly all familial cases of cerebral cavernous malformation among Hispanic Americans have been linked to a founder mutation of CCM1 localized to 7q (KRIT1 gene) 4. They can occur throughout the brain but are most common in the subcortical Rolandic and temporal areas. 5. Clinical presentation can include hemorrhage, seizures, and/or progressive neurologic deficits. Annual bleeding rates are up to 1% per year for supratentorial lesions and up to 3% per year for brainstem lesions. 6. Cavernous malformations are typically identified on MRI (“popcorn-like lesions”) and are often angiographically occult. 7. Asymptomatic cavernous malformations are followed without intervention. Surgical resection may be indicated for progressive neurologic deficits, intractable epilepsy, and/or hemorrhage. Stereotactic radiosurgery is an option for nonoperable lesions. C. Developmental venous anomaly 1. Developmental venous anomalies (DVAs) or venous angiomas are the most common congenital vascular malformation and consist of radially arranged medullary veins. 2. Usually diagnosed as an incidental finding on MRI, DVAs are usually supratentorial and solitary but can be multiple and occur with cavernous malformations. 3. DVAs rarely present with seizures or hemorrhage. Most patients are followed without intervention. Surgery is rarely required for hemorrhage or intractable epilepsy. D. Capillary telangiectasia 1. Small lesions that are usually found incidentally on MRI, in the pons, middle cerebellar peduncles, and dentate nuclei. 2. Multiple lesions are common. They are not associated with morbidity, and intervention is not required. V. The Critical Care Management of Status Epilepticus A. General principles of status epilepticus 1. Status epilepticus (SE) is a major neurological emergency that, similar to stroke, should be managed in a very timely manner. 2. The official definition of SE is in a controversial state; however, most neurologists agree that SE is a serious condition characterized by the presence of electrographic seizures with more than 30 minutes of uncontrolled seizure activity. 3. As the duration of seizures increases, so does the number of neurons lost, and the likelihood of a successful treatment decreases. 4. If true seizure activity occurs for longer than 5 to 10 minutes, the likelihood of cessation of activity is unlikely without intervention. 5. Convulsive SE is seemingly more serious because there are a number of body systems involved; however, increasing evidence is showing that nonconvulsive SE may also necessitate early treatment, as some neuronal loss due to SE may be irreversible. 6. The strongest predictor of outcome is cause and duration of the SE. 7. Mortality is 10% to 20% in SE, and up to 32% in refractory cases. B. Continuous EEG (cEEG) monitoring 1. EEG has been used in medical ICUs for decades to assess for nonconvulsive seizures in the comatose population and to further clarify alterations in mental status. 2. Technological improvements have allowed for the use of cEEG monitoring in the hospital. As the number of patients on cEEG monitoring increased, so did the detection of subclinical seizure activity. 3. The incidence of seizures in the neurological ICU patient population has been found to be roughly around 30%. C. Common types of status epilepticus treated in the neurological ICU setting 1. Generalized convulsive status epilepticus (GCSE): continuous seizure activity associated with generalized tonic-clonic convulsions for an indefinite amount of time; tends to increase lactate and creatine kinase levels in the blood, and can elevate whole body temperature, which all can lead to further complications. 2. Nonconvulsive status epilepticus (NCSE): continuous seizure activity, most often noted on EEG record, without typical manifestations of motor activity as in GCSE; generally presents as an altered mental state 3. Simple-partial status epilepticus: Also known as epilepsy partialis continua (EPC), an electrographic phenomenon that can cause motor or sensory symptoms or alteration in mental status, or a combination of symptoms generally lateralized and/or localized to a certain brain area without spread to other brain regions. Because of their localization, these seizures generally do not affect awareness. D. Managing SE in the ICU 1. Treatment initiation of SE in an expeditious manner is of utmost importance to improve outcomes. 2. Often the ABC (airway, breathing, circulation) method of therapy is indicated for SE patients upon arrival to secure the patient’s airway and to provide immediate supportive care. 3. The management of SE in the ICU centers on stopping the electrographic seizures and determining etiology while avoiding complications and preventing recurrence. a. Acute seizure management i. Imaging should be completed as soon as possible, and a cEEG monitor should be placed on the patient immediately upon the suspicion of SE. ii. Initially benzodiazepines (IV lorazepam, IM midazolam, PR diazepam) are most useful as first-line pharmacological abortive therapies. iii. The clinician should be prepared to infuse fosphenytoin (fPHT) or valproic acid (VA) within the first few minutes if the seizures are refractory to benzodiazepine therapy. iv. Levetiracetam (LEV) and lacosamide (LCM) can be considered for abortive therapy; however, evidence for their use is currently lacking. v. Should the SE be refractory to fPHT or VA, consideration for anesthetic doses of propofol, phenobarbital, or midazolam should be made (mainly in cases of NCSE and GCSE). vi. Pentobarbital should be reserved for refractory cases. vii. Ketamine is also being researched as an SE abortive therapy. b. Complication management i. Patients with SE may be hypertensive; however, hypertension in the acute setting of SE rarely needs to be treated because anti-epileptic medications generally cause a drop in blood pressure. ii. The rise in creatine kinase and rhabdomyolysis, however, pose a threat to other organ systems and should be monitored. iii. Other ICU complications such as DVT, ventilator-associated pneumonia, catheter-associated urinary tract infection, and bloodstream infection should be monitored and treated accordingly. iv. Propofol-related infusion syndrome (PRIS) is a life-threatening condition characterized by acute refractory bradycardia progressing to asystole and one or more of the following: (1) metabolic acidosis, (2) rhabdomyolysis, (3) hyperlipidemia, (4) enlarged or fatty liver. Large dose for a long time (>4 mg/kg/hr for 48 hours) is a risk factor. c. Etiology management: the etiology of SE should be ascertained as soon as the seizure management algorithm is completed. The following SE etiologies are more commonly seen in the neurological critical care setting: i. Bacterial meningitis: treated with empiric antibiotics followed by de-escalated antibiotic therapy based on cultures. Steroid management can help, but its use is controversial. Continuation of AED is important while treating the underlying bacterial infection. ii. Ethyl alcohol (ETOH) withdrawal: generally seen no earlier than 24 to 48 hours since the last alcoholic drink; treat promptly with benzodiazepine or barbiturate agent. Phenytoin has not been shown to be very efficacious for ETOH withdrawal seizures. iii. Viral meningitis/encephalitis: generally secondary to Herpes simplex virus (HSV); treat with acyclovir and standard generalized anti-epileptic medications. iv. Autoimmune encephalitis: There are a number of autoimmune etiologies to SE (both paraneoplastic and idiopathic). Among them are limbic encephalitis and autoimmune encephalitis. Treatment is related to immune suppression combined with plasmapheresis and/or intravenous immunoglobulin therapy and steroids in addition to escalated anti-epileptic medication therapy. There is no current standard therapy for autoimmune-related encephalitis. VI. Brain Herniation A. Herniation syndromes B. “Neuro coding”: The immediate treatment of herniation syndromes and/or high ICP through the use of the following: 1. Hypertonic saline a. 23.4% saline “bullet” (central line only) b. 3% saline bolus (central line only) c. 2% saline bolus (peripheral or central line) 2. Mannitol push (0.5– 1g/kg) 3. Hyperventilation 4. Elevation of the head of the bed 5. Pentobarbital or propofol VII. Coma and Brain Death A. Coma: There are some subtle semantic differences in the exact definition of coma; however, most describe a coma as a profound unarousable state of unresponsiveness and unconsciousness with little or no response to pain, voice, or other external stimulation and no spontaneous eye opening. 1. As such, the Glasgow Coma Scale (GCS) and the Full Outline of UnResponsiveness (FOUR) score were created to assess the level of responsiveness and awareness. 2. Etiologies for coma include severe bilateral hemispheric brain damage, injury to vital areas of the reticular activation system (brainstem, basal forebrain, thalamus and hypothalamus), and metabolic, septic, or drug effect. 3. It is important to note that arousal requires an intact brainstem and subcortical function, whereas awareness requires an intact cerebral cortex. 4. Management of coma requires standard airway, breathing, and circulation supportive therapy while determining etiology and the potential for reversibility. 5. FOUR score PATTERN SCORING Eye response 4—Eyelids open, tracking or blinking 3—Eyelids open, but not tracking 2—Eyelids closed, but opens to loud voice 1—Eyelids closed, but opens to pain 0—Eyelids closed, even with pain Motor response 4—Thumb’s up, fist, or peace sign to command 3—Localizing to pain 2—Flexion to pain 1—Extension to pain 0—No movement to pain or generalized myoclonus status Brainstem reflexes 4—Pupil and corneal reflexes present 3—One pupil wide and fixed 2—Pupil or corneal reflex absent 1—Pupil and corneal reflex absent 0—Absent pupil, corneal, and cough reflex Respiration 4—Not intubated, regular breathing pattern 3—Not intubated, Cheyne-Stokes breathing pattern 2—Not intubated, irregular breathing pattern 1—Intubated, breathes above ventilator rate 0—Intubated, breathes at ventilator rate or apnea 6. Glasgow Coma Scale Eye opening Spontaneous 4 To voice 3 To pain 2 None 1 Best motor response Obeys commands 6 Localizes pain 5 Withdraws to pain 4 Flexor posturing 3 Extensor posturing 2 None 1 Best verbal response Conversant and oriented 5 Conversant and disoriented 4 Inappropriate words 3 Incomprehensible words 2 None 1 7. Respiratory patterns associated with coma PATTERN DESCRIPTION LOCATION Cheyne-Stokes Hyperpnea (in a crescendo-decrescendo pattern) followed by apnea Bilateral diencephalon or cerebrum Central neurogenic hyperventilation Regular, rapid, deep hyperpnea Brainstem tegmentum Apneustic Hyperpnea pauses at full inspiration Pons Ataxic Irregular rate and depth of respiration Medullary reticular formation 8. Pupillary clues in the comatose state DESCRIPTION LESION Small and reactive Diencephalon Ipsilateral pupillary constriction Hypothalamus Pinpoint but still reactive Pons Large and fixed Tectum Midposition and fixed Midbrain Dilated and fixed Uncal herniation B. Brain death 1. The difference between coma and brain death is that brain death is characterized by the total irreversible loss of all brain function to sustain life. 2. The term serves as a second legal declaration of death, the only other currently being cardiac death. Certain conditions must be met to make the declaration of death by neurological criterion: a. The cause must be known and must be irreversible. b. There must be no severe overlying medical condition (electrolytes, acid/base disturbances, endocrine abnormalities). c. There must be no drug intoxication or poisoning. d. The core temperature of the patient must be at least 90°F (32°C). e. There should be no recent administration or continued presence of neuromuscular blocking agents. 3. The neurological examination must demonstrate the following: a. The patient must be completely unresponsive (no motor response to pain). b. The patient must exhibit complete loss of brainstem reflexes. i. No pupillary response to light ii. No oculocephalic reflex (doll’s eyes maneuver) iii. No caloric vestibular reflex (“cold calorics”): using 50 mL of cold water, instill water for 1 minute into each ear and allow for at least 5 minutes between ears. Eyes should turn slowly toward the ear being stimulated in the patient with intact reflexes. iv. No corneal reflex; no grimacing to pain v. No gag reflex; no cough or bradycardia with suction c. The patient must not be spontaneously breathing. To test for spontaneous breathing, an apnea test may be completed as follows: i. Patient’s body temperature must be at least 97°F (36.5°C); systolic BP at least 90 mmHg; no diabetes insipidus or positive fluid balance in the past 6 hours. ii. Patient should be preoxygenated to achieve a PO2 of at least 200 and PCO2 of at least 40 or lower. Cannulated 100% oxygen can be delivered down the endotracheal tube to provide oxygenation. iii. The ventilator should be disconnected once the previous steps are complete. Patient will pass the apnea test (not deemed brain dead) if there are any respiratory movements. The apnea test should be stopped if the systolic BP drop to less than 90 mmHg, if the PO2 significantly decreases, or there is SpO2 desaturation or cardiac arrhythmia. iv. Serial arterial blood gases should be drawn while ventilator is disconnected: test is positive for brain death (patient fails apnea test) if PCO2 rises to 60 mmHg or there is a 20-mmHg increase over baseline; generally should not take longer than 8 minutes. 4. If the patient meets all of the previous criteria, generally on two separate occasions at least 6 hours apart, patient meets the legal definition of death by neurological criteria posed by most states. The apnea test only needs to be completed once over the two occasions. There are several confirmatory tests that can assist with the clinical examination if there is uncertainty of brain death: a. Digital subtraction angiography can be done, which in the brain-dead patient will demonstrate no filling above the level of carotid bifurcation (internal carotid artery), the intracranial vertebral arteries, or the circle of Willis. b. Electroencephalography: Brain death is indicated by electro-cerebral silence noted with no EEG activity over 2 uV when recording from scalp electrode pairs 10 cm or more apart with interelectrode impedances between 100 and 10,000 Ω. A minimum of eight electrodes is necessary. There should also be no electroencephalographic reactivity to somatosensory or audiovisual stimuli. c. Transcranial Doppler ultrasound: total cerebral circulatory arrest will be seen in the patient with brain death. This will be indicated by a lack of diastolic flow and small sharp systolic peaks in early systole. Sensitivity is greater than 95% and specificity is 100% for brain death. d. Technetium 99 hexamethylpropyleneamine oxime brain scan: this test is very sensitive and specific. It will show no radiotracer uptake within the cerebral vessels or parenchyma in the brain-dead patient. e. Somatosensory evoked potentials: no response of N20 to P22, indicating a lack of communication between the body and the cortex.
(bright)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




