CHAPTER 113 NEUROLOGY OF CARDIOLOGY
The neurology of cardiology may be divided into three categories: (1) the cardiac complications of neurological disease; (2) the neurological complications of cardiac disease (e.g., cardiac-source embolic stroke, neurological complications of cardiac surgery); and (3) neurocardiac syndromes (mitochondrial diseases; Friedreich’s ataxia; muscular dystrophies affecting the heart). The latter two categories are covered in other chapters of this book. This chapter deals with the cardiac manifestations of neuropsychiatric illnesses.
In 1942, Walter Cannon reported several incidents of “voodoo” death,1 by which he meant death from fright. These events had several features in common. They were all induced by an absolute belief that an external force could cause demise and that the victim had no power to alter this course. Cannon postulated that death was caused by intense action of the sympathetic adrenal system. Evidence has accumulated to support the concept that voodoo death is a real phenomenon and is not limited to ancient peoples. Rather, it may be a basic biological principle that provides an important clue to understanding the phenomenon of sudden death in modern society, as well as opening a window into the world of neurovisceral disease. George Engel collected 160 accounts from the lay press of sudden death that was attributed to disruptive life events2 and concluded that such events could be divided into eight categories (Fig. 113-1), the common feature of which is life-threatening stress without escape or control.
Richter reported a series of experiments aimed at elucidating the mechanism of “voodoo” death.3 He found that domesticated rats could swim for about an hour in 93°F water, but if the animals’ whiskers were trimmed, they would invariably drown within a few minutes. When similar experiments were performed with wild rats, restraint contributed significantly to the tendency for demise, whereas, in the case of the calm, domesticated animals in which restraint and confinement were apparently not significant stressors, shaving the whiskers rendered these animals as fearful as wild rats, with a corresponding tendency for sudden death. Adrenalectomy did not protect the animals.
In humans, one of the easily accessible windows into autonomic activity is the electrocardiogram (ECG). Byer and associates reported six patients with neurological disease in whom ECGs showed large upright T waves and long Q-T intervals.4 On the basis of experimental results of cooling or warming the endocardial surface of the dog’s left ventricle, they concluded that these electrocardiographic changes resulted from subendocardial ischemia. Levine reported a patient with a subarachnoid hemorrhage who had electrocardiographic changes reminiscent of coronary disease.5 Burch and colleagues reported 17 patients with various types of stroke who had long Q-T intervals, large and usually inverted T waves, and frequent U waves.6 Cropp and Manning reported the abnormalities on ECGs in 29 patients with subarachnoid hemorrhage; in five of these patients, autopsy study verified the absence of coronary artery disease and myocardial infarction, which suggested that the abnormalities on ECGs were neurogenic.7
Selye described electrolyte-steroid cardiopathy with necrosis and argued that this lesion was distinct from the coagulation necrosis that occurred as a result of ischemic disease.8 Certain steroids and other hormones created a predisposition for the development of electrolyte-steroid cardiopathy with necrosis, but other factors were necessary for its development, including stress. Raab and associates found that cardiac lesions may be produced in rats by pretreatment with fluorocortisol, calciferol, or thyroxine, followed by restraint or cold stress, and that pharmacological blockade of sympathetic activity was cardioprotective.9 Intracoronary infusions of adrenaline reproduce the characteristic electrocardiographic pattern of neurocardiac disease, which is reminiscent of subendocardial ischemia, although no ischemic lesion could be found in the hearts of dogs sacrificed after several months of infusions.10
Melville and coworkers produced changes on ECG and myocardial necrosis by stimulating the hypothalamus of cats; autopsy studies revealed evidence of open coronary arteries.11 The cardiac lesion was characterized by intense cytoplasmic eosinophilia with loss of cross-striations and some hemorrhage, a condition now most commonly known as contraction band necrosis. Oppenheimer and Cechetto mapped the chronotropic organizational structure in the rat insular cortex, demonstrating that sympathetic innervation arises from a more rostral part of the posterior insula than does parasympathetic innervation.12
Despite the fact that myocardial damage had been produced in animals, it was not until Koskelo and associates reported on three patients with electrocardiographic changes caused by subarachnoid hemorrhage13 that contraction band necrosis was demonstrated in humans with neurological disease. Connor reported focal myocytolysis in 8% of 231 autopsy studies; the incidence was highest in patients who had died of intracranial hemorrhages.14 Connor pointed out that prior pathological reports probably overlooked the lesion because of the fact that it was multifocal, each individual focus being quite small, necessitating extensive tissue sampling. It is clear now that even Connor underestimated the prevalence of the lesion and that serial sections are required to rigorously rule out its presence. Greenshoot and Reichenbach reported nine patients with subarachnoid hemorrhage, all of whom had cardiac lesions, and demonstrated that the cardiac pathology could be reproduced in cats given mesencephalic reticular formation stimulation.15 Adrenalectomy did not protect the hearts. This finding supported the contention that the electrocardiographic changes and cardiac lesions result from direct intracardiac release of catecholamines. Hawkins and Clower injected blood intracranially into mice, thereby producing the characteristic myocardial lesions, which could be ameliorated but not prevented by pretreatment with adrenalectomy.16 This finding indicated that humorally delivered catecholamines had some role in the genesis of neurocardiac necrosis.
Jacob and colleagues produced subarachnoid hemorrhage experimentally in dogs and carefully studied the sequential hemodynamic and ultrastructural changes that occurred.17 The hemodynamic changes occurred in four stages, directly corresponding to the effects seen with intravenous noradrenaline injections: hypertension, tachycardia, rise in left ventricular pressure, and increased coronary blood flow. Ultrastructurally, a series of three stereotyped events, which could be imitated exactly with noradrenaline injections, occurred: migration of calcium-containing granules to the periphery of mitochondria, disappearance of these granules, and myofilament disintegration at the I bands. Partially successful efforts to modify the developments of neurocardiac lesions were made by McNair and coworkers, using reserpine pretreatment in mice subjected to simulated intracranial hemorrhage,18 and by Hunt and Gore, who pre-treated a group of rats with propranolol and then attempted to produce cardiac lesions with intracranial blood injections.19
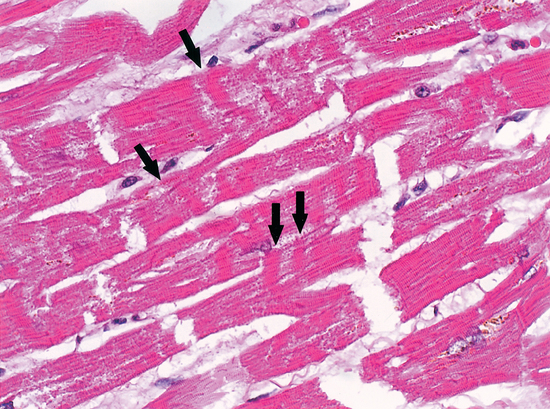
The phenomena of the various types of myocardial cell death were clarified by Baroldi,20 who described three patterns: coagulation necrosis, the fundamental lesion of infarction; colliquative myocytolysis, the fundamental lesion of low output syndromes; and coagulative myocytolysis (now known as contraction band necrosis), the fundamental lesion of catecholamine-induced necrosis.
Contraction band necrosis may be seen in reperfused areas around regions of coagulation necrosis, in sudden unexpected and accidental death, and in hearts exposed to high levels of catecholamines, as in people with pheochromocytoma. This is probably the major lesion described by Selye as electrolyte-steroid cardiopathy with necrosis8 and is clearly the lesion seen in animals and people suffering acute neurological or psychiatric catastrophes (Fig. 113-2).
It is likely that the subcellular mechanisms underlying the development of contraction band necrosis involve calcium entry. Zimmerman and Hulsmann reported that the perfusion of rat hearts with calcium-free media for short periods creates a situation such that upon readmission of calcium, there is a massive contracture, followed by necrosis and enzyme release.21 This phenomenon, known as the calcium paradox, can be imitated almost exactly with reoxygenation after hypoxemia and reperfusion after ischemia. The latter, called the oxygen paradox, has been linked to the calcium paradox by pathological calcium entry.22 This major ionic shift is probably the cause of the dramatic changes seen on ECG in the context of neurological catastrophe, a fact that could explain the phenomenon of sudden unexpected death in many contexts: for example, sudden death in middle-aged men; sudden infant death syndrome; sudden unexpected nocturnal death syndrome; frightened to death (“voodoo” death); sudden death in epilepsy; sudden death during natural catastrophe; sudden death associated with drug abuse; sudden death in wild and domestic animals; sudden death during asthma attacks; sudden death during the alcohol withdrawal syndrome; sudden death during grief after a major loss; sudden death during panic attacks; sudden death from mental stress; and sudden death during war. The connection between the nervous system and the cardiopulmonary system provides the unifying link that allows a coherent explanation for most, if not all, of the forms of sudden unexpected death. Powerful evidence from multiple disparate disciplines allows for a neurological explanation of sudden unexpected death.23
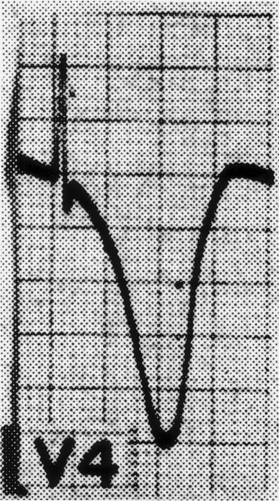
A wide variety of changes in the ECG is seen in the context of neurological disease. Two major categories of change are arrhythmias and repolarization changes. It is likely that the increased tendency for life-threatening arrhythmias found in patients with acute neurological disease arises from the repolarization change, which increases the vulnerable period during which an extrasystole would be likely to result in ventricular tachycardia and/or ventricular fibrillation. Thus, the essential and potentially most lethal electrocardiographic features, which are known to change in the context of neurological disease, are changes in the ST segment and T wave, reflecting abnormalities in repolarization. Most often, the changes are observed best in the anterolateral or inferolateral leads (Fig. 113-3). The electrocardiographic abnormalities usually improve, often dramatically, with brain death.
< div class='tao-gold-member'>