CHAPTER 115 NEUROLOGY OF HEMATOLOGY
THE ANEMIAS
Iron deficiency from chronic blood loss is the most common form of anemia. Iron deficiency in the absence of anemia (sideropenia) may decrease the deformability of red blood cells, leading to ischemia in the distribution of small cerebral vessels. This mechanism is particularly important in the context of polycythemia, in which there are increased numbers of red blood cells, each one of which may be iron deficient. Both the polycythemia and the relative sideropenia lead to increased blood viscosity with associated neurological symptoms and signs. Iron deficiency causes a microcytic, hypochromic anemia.
The restless legs syndrome is a very common cause of insomnia. It consists of an unpleasant creeping sensation that occurs deep in the legs (and occasionally in the arms) when the person is at rest. The person feels compelled to move the legs to avoid the unpleasant feeling. Most sufferers are women, who pace the floor at night and complain of insomnia. Polysomnographic studies often reveal nocturnal myoclonus. It is likely that restless legs syndrome and nocturnal myoclonus represent various fragments of a single disorder, known as Ekbom’s syndrome.1 Many of the movement disorders associated with iron deficiency are reminiscent of those seen in basal ganglia diseases, but the precise relationship between systemic iron deficiency and these movement disorders is cryptic, although there is some reason to believe that iron is a cofactor for the enzyme tyrosine hydroxylase, which catalyzes the rate-limiting step in the biosynthesis of dopamine from tyrosine. Ekbom’s syndrome may therefore be a dopamine deficiency syndrome distinct from parkinsonism. Patients with Ekbom’s syndrome may respond to iron replacement. The rest are treated with centrally acting dopamine agonists, such as pramipexole or ropinirole. Some patients who fail to respond to a dopamine agonist derive benefit from a benzodiazepine, clonidine, or clomipramine. Patients diagnosed as having the restless legs syndrome should undergo careful evaluation for anemia, including microscopic study of the blood smear, measurements of serum iron and total iron-binding capacity, and several stool tests for occult blood. Blood and spinal fluid ferritin levels may be assayed in patients without overt evidence of iron deficiency.
Disordered DNA metabolism is clearly not confined to the blood cells, inasmuch as giant epithelial cells are found in many other organs, including the mouth, stomach, and skin. The neurological effects of the megaloblastic anemias probably result from a primary metabolic derangement in neural tissue and are clearly not directly related to the anemia per se.2 Because the blood-forming organs are particularly sensitive to the effects of cobalamin or folate deficiency, it is unusual to find the neurological effects in patients in whom no disorders of the blood are found. Anemia is, however, only one and probably a relatively late sign of cobalamin or folate deficiency, and so it is possible to find patients with the neurological effects of cobalamin or folate deficiency without anemia.3
Cobalamin (vitamin B12) deficiency may have a number of causes, including (1) defective diet (low in animal or bacterial products), (2) defective absorption (deficiency of intrinsic factor as a result of pernicious anemia, gastrectomy, or intestinal disease such as malabsorption or “blind loop” syndrome), and (3) increased metabolism (thyrotoxicoxis, pregnancy, neoplasia). Of these, the most prevalent form of cobalamin deficiency is pernicious anemia. It arises from failure of the gastric fundus to secrete adequate amounts of intrinsic factor to ensure intestinal absorption of vitamin B12. This failure of secretion of the mucoprotein intrinsic factor is caused by atrophy of the fundic glandular mucosa, a process that is usually an immune system–mediated gastritis but may be familial or senile or may result from gastric neoplasia. The presence of histamine-fast achlorhydria is a reliable method of diagnosing pernicious anemia but has often been supplanted by measurements of anti-intrinsic factor and antiparietal cell antibodies.4 Patients with autoimmune pernicious anemia often have clinical and laboratory evidence of other conditions characterized by autoimmunity, such as vitiligo and thyroiditis. Serum B12 levels have occasionally been found to be erroneously normal in documented cases, and so it is now routine to assess intracellular function by directly measuring serum homocysteine (for folate or cobalamin deficiency) and methylmalonic acid (for cobalamin deficiency).
Exposure to nitrous oxide, as in the induction of general anesthesia, may precipitate acute deterioration (anesthesia paresthetica) in patients with otherwise mild or asymptomatic cobalamin deficiency.5 The adenosylcobalamin system acts to metabolize propionic acid by converting methylmalonyl–coenzyme A to succinyl–coenzyme A. Failure of this system results in an accumulation of methylmalonic acid, which is myelinotoxic by promoting the formation of abnormal long-chain fatty acids.
Subacute combined degeneration of the spinal cord is the term used to designate the spinal cord disease caused by cobalamin deficiency.6 Patients complain of generalized weakness and paresthesias that usually begin distally in the hands. As these symptoms progress, stiffness and weakness in the limbs develop. Loss of vibration sense is the most profound sign, often joined later in the course by joint position sense loss. The Romberg sign is positive, and the gait is unsteady and awkward primarily because of proprioceptive loss (pseudotabetic gait). Weakness and spasticity are usually worse in the legs than in the arms and may progress to a spastic paraplegia if untreated. Babinski’s signs are present, but the deep tendon reflexes are variable. If a sensory level implicating the spinothalamic tracts is found on the trunk, this should always be viewed with the greatest skepticism, and the clinician should exhaustively rule out other causes of spinal cord disease. Many patients with vitamin B12 deficiency have distal symmetrical impairment of cutaneous sensation, absence of deep tendon reflexes, and even slowed nerve conduction velocities, which suggest a neuropathic component, but this is usually quite mild compared to the myelopathic illness. These manifestations may sometimes be visualized by magnetic resonance imaging and pathologically are seen as regions of spongy myelopathy, quite similar to those seen in human immunodeficiency virus–related myelopathy, raising the question of whether this virus may somehow impair the transmethylation function of cobalamin.
Optic neuropathy is the third and last major neurological complication of vitamin B12 deficiency. It is characterized by bilateral involvement of the optic nerves that results in loss of central visual acuity and depressed sensitivity, more so for color than for black and white, in the centrocecal area of the field of vision. This syndrome is clinically similar to a number of other bilateral optic neuropathy syndromes, including tobacco-alcohol amblyopia, diabetic optic neuritis, Leber’s hereditary optic atrophy, and tropical ataxic neuropathy. These syndromes may be linked to an abnormality in cyanide metabolism that results from a shortage of sulfur-donating amino acids. In the 1990s, there was an epidemic of optic neuropathy and myelopathy in Cuba, thought to be caused by multiple B-vitamin deficiency resulting from malnutrition in combination with alcohol and cyanide exposure from cigar smoking and cassava consumption. The epidemic was terminated by vitamin supplementation.7
Folic acid (folate) deficiency accounts for nearly all of the cases of megaloblastic anemia not caused by vitamin B12 deficiency. The causes of folate deficiency are (1) defective diet (low in vegetables and liver), (2) defective absorption from intestinal malabsorption as a result of sprue, steatorrhea, diverticulosis, or short circuits of the gastrointestinal tract or the “blind loop” syndrome; and (3) deranged metabolism caused by an increased requirement from hemolytic anemia, pregnancy, or neoplasia or by impairment of use as a result of liver disease or the administration of folic acid antagonists or anticonvulsants. Unlike those of vitamin B12, the bodily stores of folic acid are quite limited. A folate deficiency syndrome may commence within several months of dietary deprivation, which makes it a much more common problem among the malnourished than is vitamin B12 deficiency. Folate, once absorbed through the entire small intestine, is reduced by specific liver enzymes to tetrahydrofolic acid, a compound that plays a major role in the metabolism of one carbon fragments by its synthesis and transfer of methyl groups. Through this mechanism, folate is vital for the conversion of deoxyuridylate to thymidylate, a precursor needed for DNA synthesis. Thus, tetrahydrofolate derivations are closely linked to vitamin B12–dependent reactions, and the hematological alterations in vitamin B12 and folate deficiency are indistinguishable. Deficiencies of the two vitamins have very similar effects, and a deficiency of one may lead to faulty use of the other. Many patients with vitamin B12 deficiency have concomitant folate deficiency, but most people with the folate deficiency state, which is overwhelmingly more common, have no vitamin B12 deficiency. Folic acid deficiency is almost never pure. Because it accompanies malnutrition, it is nearly always associated with multiple vitamin deficiencies. The most common neurological manifestation of this multivitamin deficiency state is a symmetrical sensorimotor polyneuropathy. Some minor degrees of segmental demyelination may also occur, usually as a result of entrapment of metabolically weakened nerves. All the common entrapment neuropathies (e.g., carpal tunnel syndrome, meralgia paresthetica, peroneal palsy, ulnar palsy) are more frequent in patients with an underlying metabolic axonopathy such as that caused by vitamin deficiency. Folate deficiency is associated with neural tube defects, and supplements are therefore routinely prescribed during pregnancy.
THE HEMOGLOBINOPATHIES
Painful crises are among the most common clinical problems in the management of patients with sickle cell anemia. The abdominal and bone pain, so common in this disease, is probably ischemic pain related to the sickling phenomenon. The treatment for these crises consists of hydration, bed rest, and analgesia. Vascular disease is the more serious neurological aspect of this disorder and probably contributes in a major way to the decrease in life expectancy of patients with sickle cell anemia. The prevalence of overt strokes is about 20% among patients with sickle cell anemia. Most strokes are caused by small-vessel occlusions, which often result in seizures at the onset of a stroke. In some cases, progressive small-vessel occlusions with recurrent development of collateral vessels can lead to an angiographic picture similar to that seen in moyamoya disease. Hemorrhages may result from rupture of these fragile collateral vessels, leading to intracerebral, subarachnoid, spinal, and retinal hemorrhagic strokes in patients with sickle cell disease. The progressive stenosis of the supraclinoid internal carotid artery that leads to the development of the moyamoya pattern may be detected noninvasively with transcranial Doppler ultrasonography. Stroke risk is reduced dramatically through the use of prophylactic transfusions in patients in whom transcranial Doppler examinations reveal that the time-averaged mean blood velocity in the internal carotid or middle cerebral artery is 200 cm per second or higher.8 Among children younger than 15 years with strokes, sickle cell anemia is present in 7%, and thus it an important cause of stroke in childhood. Spinal cord infarction is also observed in patients with sickle cell anemia much more commonly than in the general population. Massive intracranial hemorrhage is another complication of sickle cell anemia. Large-vessel occlusions also occur in patients with the supraclinoid carotid as the site of predilection. Moyamoya disease may be treated with extracranial-intracranial arterial bypass grafting or with temporal-pial synangiosis, with the hope of reducing the likelihood of rupture of the fragile vessels of moyamoya disease.
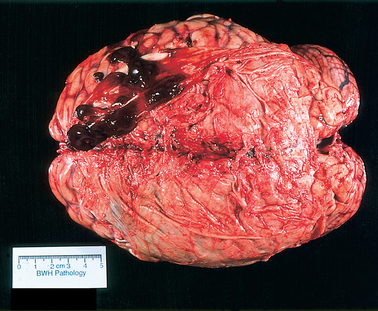
The genetic defect underlying thalassemia involves rates of synthesis of the individual polypeptide chains. Two major varieties of thalassemia exist: one involving defective α-chain synthesis, the other involving β-chain synthesis. The more common β-thalassemia may occur in the heterozygous or homozygous form to produce the syndromes of thalassemia trait or Cooley’s anemia (thalassemia major), respectively. Heterozygosity for α-thalassemia results in a very mild condition and may require an associated hemoglobin abnormality for clinical expression (thalassemia minor). Homozygous α-thalassemia is thought to be incompatible with normal fetal development. The susceptibility to infection seen in thalassemia corresponds to that seen in sickle cell anemia but is confined to patients who have undergone splenectomy for control of hemolysis. In about a third of patients with myelopathy resulting from extramedullary hematopoiesis, thalassemia is the underlying disease. The usual location for extramedullary hematopoiesis is various parts of the reticuloendothelial system, particularly the liver, spleen, and lymph nodes. However, the spinal epidural space and the intracranial subdural space (Fig. 115-1) may be involved, with consequent compression of the spinal cord or the brain or both. Most cases involving the spine occur in the thoracic segments posteriorly, usually over multiple levels. Treatment with radiotherapy is usually quite effective.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree