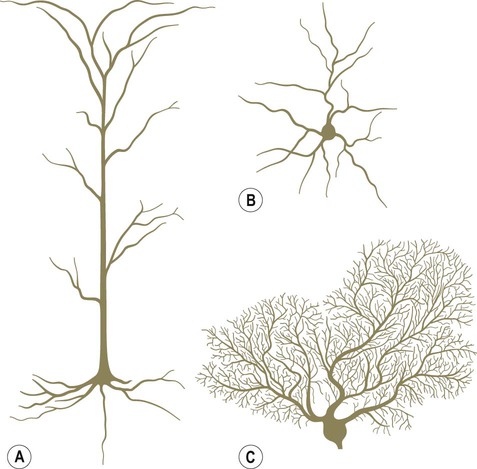
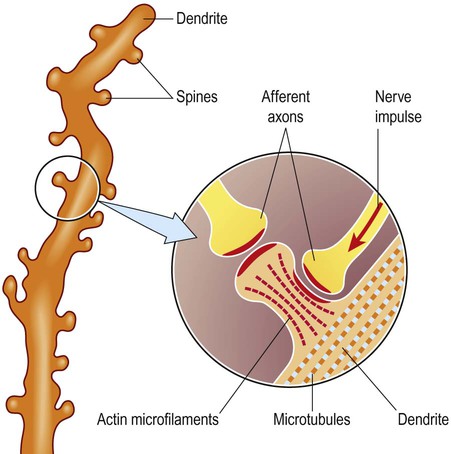
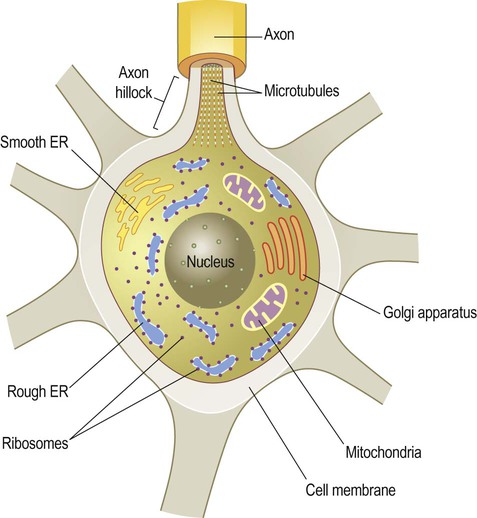
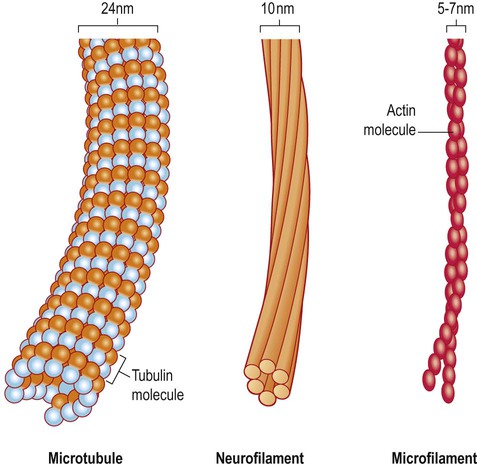
The basic structure of the neuron has been outlined in Chapter 1. It was also shown how nerve cells can be classified based upon the type of information that they transmit (afferent, efferent, interneuron) or by the number of processes that they have (unipolar, bipolar, multipolar). Neuronal excitability, impulse conduction and synaptic transmission are discussed in Chapters 6 and 7. Pyramidal cells have large, pyramid-shaped cell bodies that range from 20–120 µm in diameter. They are excitatory neurons that have numerous apical and basal dendrites and a single axon that projects out of the cortex. Pyramidal cells are particularly prominent in motor and premotor areas. Granule cells (or stellate cells) are star-shaped multipolar neurons that have short axons and make local synaptic contacts, tending to be enriched in sensory cortices. They are much smaller than pyramidal cells, with a typical diameter of less than 20 µm, and may be excitatory or inhibitory. The cerebellar cortex also contains two main types of nerve cell: granule cells (similar to those in the cerebral cortex) and Purkinje cells (large efferent neurons, equivalent to cortical pyramidal cells; see Fig. 5.1C). More than 90% of the cerebral cortex has a characteristic six-layered structure that appeared with the evolution of the mammalian brain (Fig. 5.2). For this reason it is referred to as neocortex (Greek: neos, new). Although the same six layers can be identified in all neocortical regions at some stage of development, they are not always present in the mature brain. For instance, the motor and premotor areas of the frontal neocortex are referred to as agranular cortex since they have lost their internal granule cell layer. The cerebral cortex can be divided into more than fifty Brodmann areas based on subtle differences in the cortical structure (referred to as cytoarchitectonics) but there are three major cortical types (Fig. 5.3): The majority of the ‘non-neocortical’ regions belong to the limbic lobe and are primarily concerned with emotion, memory and olfaction (the sense of smell). The term paralimbic cortex is used to describe non-neocortical regions outside of the limbic lobe proper, including the posterior orbital cortex, anterior insula and temporal pole. The dendrites of many neurons are studded with thousands of tiny, mushroom-shaped dendritic spines (Fig. 5.4). These include the medium spiny neurons that make up 95% of cells in the basal ganglia. In the cerebral cortex, all pyramidal cells have dendritic spines, whereas stellate cells may be spiny or smooth. Each dendritic spine is the site of an incoming excitatory synapse and a typical cortical pyramidal neuron has more than 10,000 spines. They are dynamic structures that can form, change shape or disappear altogether and are thought to be important in synaptic plasticity (Greek: plastikos, able to be moulded) and learning. Long-term memories may be mediated by the growth of new spines or the strengthening and enlargement of existing ones. The neuronal cell body or soma (Greek: soma, body) contains the same organelles found in other cell types (Fig. 5.5) but the machinery for protein synthesis and gene transcription is particularly prominent (Fig. 5.6A). The perinuclear cytoplasm or perikaryon (Greek: peri, around; cyton, kernel) contains a well-developed network of rough endoplasmic reticulum, often arranged in clumps called Nissl bodies. The Golgi apparatus is also prominent and is the site of post-translational modification and sorting of proteins including ion channels, neurotransmitter receptors and membrane ion pumps. Neurons have a high rate of membrane turnover and gradually accumulate non-digestible membrane components within lysosomal residual bodies. These contain golden-brown material called lipofuscin (pronounced: lipo-FUSKIN). This so-called ‘age pigment’ accumulates over time as a by-product of membrane turnover and is also abundant in the heart and liver. Catecholamine-synthesizing neurons contain the dark brown pigment neuromelanin (Fig. 5.6B); these include the substantia nigra of the midbrain and the locus coeruleus of the pons (see Ch. 1). Neurons contain various types of membrane-bound vesicle. Neurotransmitters and neuropeptides are stored in the axon terminal (prior to release) within synaptic vesicles (see Ch. 7). Coated vesicles are derived from internalization of membrane constituents and macromolecules that have been taken up from the extracellular fluid by receptor-mediated endocytosis. All cells have a cytoskeleton composed of an internal framework of fibrillar proteins, that gives each cell its characteristic shape. This molecular scaffold is particularly important in process-bearing cells such as neurons and glia, which have a complex structure. The cytoskeleton is also involved in the transport of materials between intracellular compartments (see below). The main components include microtubules, neurofilaments and microfilaments (Fig. 5.7). Microtubules are tubular polymeric proteins that are composed of repeating subunits of alpha and beta tubulin. They are 24 nm in diameter and up to 1 mm in length. Some microtubules are stable, whereas others are dynamic and can grow or shrink by the addition or removal of tubulin subunits. Microtubules are polarized. The part that is closest to the neuronal cell body is arbitrarily termed the minus (–) end and that which is nearer to the axon terminal is designated the plus (+) end. Microtubules are stabilized by binding to a range of microtubule associated proteins (MAPs). These include the phosphoprotein tau which accumulates in various degenerative conditions including Alzheimer’s disease (Ch. 12).
Neurons and glial cells
Nerve cells
Cortical neurons
Pyramidal and granule cells (Fig. 5.1A & B)
Cortical lamination
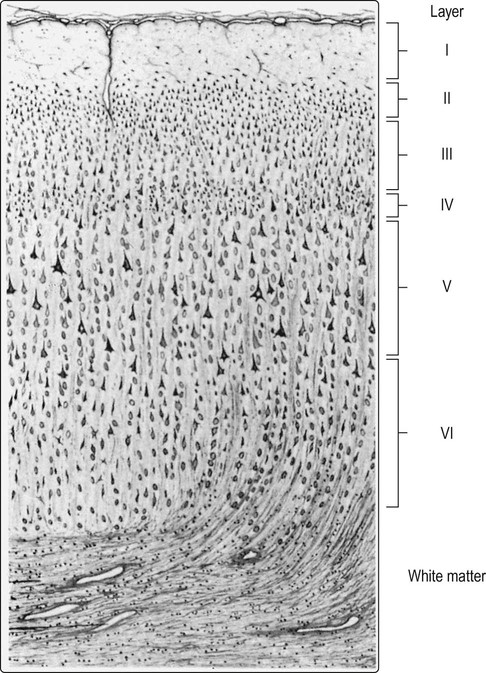
At least 90% of the cortex has six identifiable layers and is classified as neocortex. Small (granular) cells predominate in laminae that receive afferent projections, whereas large (pyramidal) cells are more numerous in layers that give rise to efferent projections. [For instance: lamina IV, the internal granule cell layer, receives projections from the thalamus; lamina V, the internal pyramidal cell layer, is the origin of projections to the brain stem and spinal cord.] From Crossman: Neuroanatomy ICT 4e (Churchill Livingstone 2010) with permission.
Different types of cortex
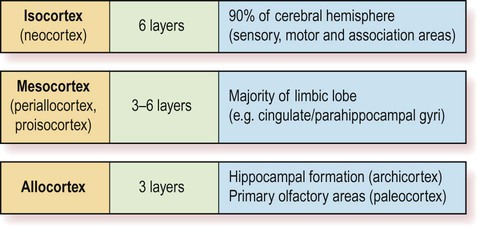
The two main types are isocortex (= neocortex) and allocortex. The mesocortex occupies a ‘transitional zone’ that lies between the six-layered isocortex and the three-layered allocortex. It has a variable number of layers and can be further subdivided into proisocortex and periallocortex, based on its similarity to the two main types.
 The isocortex has a uniform six-layered structure (Greek: isos, equal) and accounts for more than 90% of the cortical surface area. This term is synonymous with neocortex.
The isocortex has a uniform six-layered structure (Greek: isos, equal) and accounts for more than 90% of the cortical surface area. This term is synonymous with neocortex.
 The allocortex is thinner and has a more primitive, three-layered structure (Greek: allos, other). It includes the archicortex (hippocampus) and paleocortex (primary olfactory areas).
The allocortex is thinner and has a more primitive, three-layered structure (Greek: allos, other). It includes the archicortex (hippocampus) and paleocortex (primary olfactory areas).
 The mesocortex is a transitional zone between the allocortex and isocortex and has 3–6 layers. Most of the limbic lobe (cingulate and parahippocampal gyri; see Ch. 3) is composed of mesocortex.
The mesocortex is a transitional zone between the allocortex and isocortex and has 3–6 layers. Most of the limbic lobe (cingulate and parahippocampal gyri; see Ch. 3) is composed of mesocortex.
Features of the neuron
Dendritic spines
Subcellular organelles
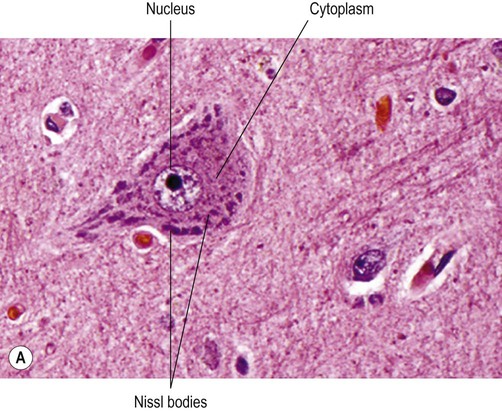
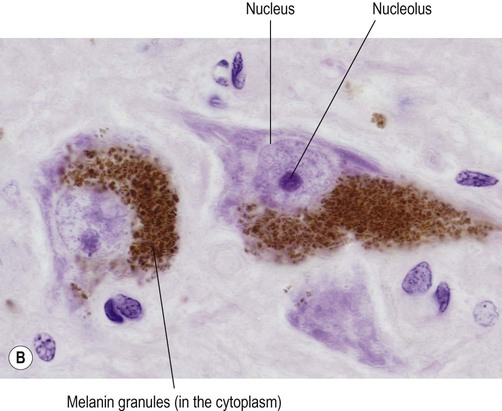
(A) Micrograph showing a pyramidal motor neuron in the cerebral cortex of the frontal lobe. It has a large nucleus with a well-defined nuclear envelope and prominent central nucleolus. The cytoplasm (stained pink) contains coarse clumps of rough endoplasmic reticulum (stained blue); these structures are referred to as Nissl bodies. [Routine haematoxylin and eosin (H&E)-stained sections.] From Prayson, Richard: Neuropathology 1e (Churchill Livingstone 2005) with permission; (B) Micrograph of two pigmented dopaminergic neurons in the substantia nigra of the midbrain (Nissl stain). The pigment is neuromelanin and is produced as a by-product of dopamine synthesis. From Standring: Gray’s Anatomy 40e (Churchill Livingstone 2008) with permission.
Lipofuscin and neuromelanin
Vesicles
The neuronal cytoskeleton
Microtubules
Axonal transport
 Anterograde fast axonal transport carries mitochondria and vesicles (e.g. enzymes for neurotransmitter synthesis) towards the axon terminal. The maximum speed is 40 cm per day.
Anterograde fast axonal transport carries mitochondria and vesicles (e.g. enzymes for neurotransmitter synthesis) towards the axon terminal. The maximum speed is 40 cm per day.
 Retrograde fast axonal transport carries worn-out mitochondria and membrane components towards the cell body (for degradation or recycling). The maximum speed is 20 cm per day.
Retrograde fast axonal transport carries worn-out mitochondria and membrane components towards the cell body (for degradation or recycling). The maximum speed is 20 cm per day.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neurons and glial cells
Only gold members can continue reading. Log In or Register to continue