16 Neurotoxicity of Chemotherapy
Types of Neurological Damage
COGNITIVE DYSFUNCTION
Cancer patients have frequently recognized decreased cognitive function (“chemo-brain”) during chemotherapy, which, in the past, was attributed by their physicians to stress or depression. Patients report problems with memory retrieval, learning, and concentration, which may persist after treatment has finished or never fully resolve.1 The incidence of acute problems during treatment ranges from 15% to 70%, with 50% of patients in one study identifying persistent problems a year after treatment.2 Cross-sectional studies also suggest persistent cognitive dysfunction in 20% to 30% of patients 2 to 10 years posttreatment.3,4
Mechanisms for this functional decline are not fully understood, but recent work by Han et al.5 on 5-fluorouracil neurotoxicity in mice suggest extensive myelin damage and persistent suppression of both oligodendrocyte and progenitor cell proliferation in the subventricular zone, hippocampus, and corpus callosum.
ACUTE ENCEPHALOPATHY
Acute encephalopathy is a common problem in oncology patients; it has a wide range of precipitating factors including metabolic derangements, hypoxia, brain metastases, meningeal carcinomatosis, infection, paraneoplastic phenomena, and drugs.6 Presenting symptoms typically include lethargy, confusion, somnolence, seizures, or coma. The diagnosis of drug-induced encephalopathy is typically one of exclusion. A careful drug history should be taken, including recent administration of narcotic analgesia and antiemetic cover, used frequently for cancer patients undergoing chemotherapy and/or for symptom control. CNS infections should also be excluded, particularly in immunocompromised and neutropenic patients. Investigations should include urea/electrolytes, liver function, serum glucose, calcium magnesium, viral serology, and CSF examination. If focal neurological deficit is present, CT or MRI imaging may be helpful and, in any case, should be undertaken prior to a lumbar puncture. EEG is particularly helpful if seizures occur, and will typically show generalized slowing with delta wave activity.7 Encephalopathy due to cytotoxic drug exposure is generally self-limiting and recovers spontaneously. The only specific therapy is the use of methylene blue in ifosfamide-induced encephalopathy, which should be considered in any patients undergoing ifosfamide chemotherapy. Risk factors include extremes of age, dose/ schedule, previous cranial radiotherapy, and renal or hepatic dysfunction.8,9
LEUKOENCEPHALOPATHY
Leukoencephalopathy may follow on from acute encephalopathy, but may also be the first indication of neurotoxicity several months to years after administration of cytotoxic drugs. Patients present with cognitive deficits, which may progress to dementia, coma, and death.10–14 MRI imaging shows widespread changes throughout the white matter,15–18 and histologically, there is axonal swelling, demyelination, and neuronal death.19 Those most at risk are patients treated with methotrexate or cytarabine, particularly if given intrathecally or if cranial radiotherapy preceded cytotoxic administration.9 Elderly patients with primary central nervous system lymphoma undergoing treatment with high-dose methotrexate and whole-brain radiotherapy (WBRT) are at particularly high risk of developing this complication.20 There is no specific therapy that can halt the progressive decline, and overall the prognosis is poor. Elderly patients with primary CNS lymphoma should be informed about these risks; it should also be considered by the treating oncologists whether these patients can be treated with reduced-dose WBRT or avoid WBRT altogether after-high dose methotrexate.
CEREBELLAR DYSFUNCTION
Cerebellar signs in an oncology patient are usually due to direct spread of cancer, particularly if it is asymmetric. In rarer situations, this can be due to paraneoplastic syndromes, which tend to have a subacute onset and may be associated with the presence of antineuronal antibodies.6 Care should also be taken to distinguish cerebellar ataxia from sensory ataxia due to a severe sensory neuropathy. Cytarabine and 5-fluorouracil are the cytotoxics most likely to cause cerebellar dysfunction including truncal ataxia, gait disturbance, and ataxia.8,14,21,22 Acutely, the MRI tends to be normal, but subsequent scans may show chronic atrophy due to irreversible Purkinje cell loss.15–18
SPINAL CORD TOXICITY
Spinal cord toxicity can occur following intrathecal administration of certain cytotoxics in acute leukemias, lymphomas, and brain tumors. Intrathecal chemotherapy is administered either as part of a lumbar puncture procedure or into the ventricles, via an Ommaya reservoir.6 The drugs most commonly used are cytarabine, methotrexate, and hydrocortisone; they may be given singly, sequentially, or together as “triple therapy.” Symptoms usually arise after multiple cycles of therapy and include both spinal cord and nerve root signs. Loss of neurological function may progress upwards.23 Histologically, there are focal areas of necrosis, particularly at the periphery of the spinal cord, associated with axonal swelling and demyelination. Myelin basic protein levels in the CSF may be elevated.24 Typically, only half of those affected will show any sign of recovery.
PERIPHERAL NEUROPATHY
Peripheral neuropathies are the most common neurological complications in patients receiving chemotherapy, especially with regimens containing taxanes (taxol, docataxel), platinum (cisplatin, carboplatin, oxaliplatin), and vinca alkaloids (vincristine). The neuropathy tends to be predominantly sensory in nature, with a glove and stocking distribution. Generally, symptoms are self-limiting, but in some patients the symptoms persist. The effect is cumulative,6 and patients frequently complain of acute subjective paresthesia of the extremities 2 to 3 days after chemotherapy. With subsequent treatment cycles, symptoms may progress to permanent paresthesia, with decreased sensation to pinprick, light touch, and vibration on formal testing.9,25–29 At this stage, chemotherapy should be stopped or the dose reduced, as continuing can lead to difficulty with activities of daily living. The neuropathy is largely reversible over several months but many patients may be left with some degree of paresthesia. In rare instances, neuropathy may be paraneoplastic in origin. Oxaliplatin is unusual in that it causes acute cold dysesthesias, as well as pharyngolaryngospasm, which usually starts shortly after administration of chemotherapy and then resolves.
Grading Toxicity
There is no universal grading system for the evaluation of patients with neurological toxicity although two neurotoxicity scoring systems are frequently used: NCI-CTC 3 (National Cancer Institute Common Toxicity Criteria version 3) and ECOG (Eastern Cooperative Oncology Group).30 The NCI-CTCAE scores a variety of symptoms from ataxia to motor neuropathy on a scale of 0 to 5, with 0 being normal, 1 mild self-limiting, 2 moderate, 3 severe undesirable, 4 life-threatening, and 5 death induced by adverse event. The full version can be downloaded from www.fda.gov. The ECOG criteria are organized in a similar manner with a scale from 0 to 4 and can be viewed at www.ecog.org. Generally patients with a toxicity score of 1 to 2 can continue with their treatment unmodified, while those with a score of 3 or 4 require dose modification or cessation of treatment.
Prevention
The patients most at risk are those receiving a high cumulative dose or intensive schedule, particularly if there is a preexisting condition such as diabetes mellitus, hereditary neuropathy, or multiple sclerosis.31 Previous radiotherapy may also increase the risk of developing neurotoxicity if patients are subsequently treated with cisplatin or methotrexate.32,33
Patients with HIV-related malignancies are also at increased risk of cytotoxic-induced neuropathy, since both HIV and the drugs used to treat it (highly active antiretroviral therapy, or HAART) can cause neurological damage independently.34 Distal sensory neuropathy is the commonest form of HIV-associated neuropathy and can be difficult to distinguish from that caused by specific nucleoside antiretrovirals. In addition, compounded neurological toxicities frequently occur because of reduced clearance of vincristine, vinblastine, and taxane; these complications can be reduced by changing these patients’ antiretroviral medications. More rarely, patients can develop inflammatory demyelinating polyneuropathy, mononeuritis multiplex, or neuronal damage due to opportunistic infections such as CMV and HZV.
Currently there are few therapies able to prevent neurological toxicity preemptively. Infusions of methylene blue are used prophylatically in patients receiving ifosfamide who have previously developed acute encephalopathy. A number of trials have investigated the benefits of agents such as calcium-magnesium infusions, carbamazepine, gabapentin, amifostine, and glutathione.7,35–52 However, the trials are small studies and no specific prophylaxis can be routinely recommended.
Treatment of Neurotoxicity
Apart from dose reduction or discontinuing the drugs implicated in the development of neurotoxicity, there is very little in the way of specific pharmacological management to reverse their side effects. Methylene blue is used for ifosfamide-induced encephalopathy and infusional calcium with magnesium may lessen the severity of established peripheral neuropathy due to oxaliplatin. However, considerable input from the multidisciplinary team is required in the holistic care of the patient,53 including analgesia for neuropathic pain, maximization of mobility (with occupational and physiotherapist involvement where there is loss of balance, sensory loss, or muscle weakness), and good nursing care to manage bowel or bladder dysfunction, protection of pressure areas in immobilized patients, and maintenance of a safe environment if patients are confused.
Cytotoxic Agents and Their Neurological Toxicities (See Table 16-1)
5-FLUOROURACIL
5-fluorouracil (5-FU), a pyrimidine analogue, is widely used in the treatment of gastrointestinal malignancies; it is administered either as a short intravenous bolus or as a prolonged continuous infusion. Neurotoxicity is rare, but may include acute cerebellar dysfunction in 3% to 7% of patients, causing gait ataxia, nystagmus, and scanning speech.54,55 Symptoms tend to resolve spontaneously within a few days of treatment cessation although administration of thiamine may be helpful.22 Patients may also develop acute confusion in the absence of cerebellar signs, which may recur on reexposure to 5-FU.56 The main risk factor for development of neurotoxicity is deficiency of the enzyme dihydropyrimidine dehydrogenase which metabolizes 5-FU.57–59
A subacute form of leukoencephalopathy occurs in approximately 2% of patients receiving 5-FU in combination with levamisole (an immunomodulatory agent).60–64 Presentation is with focal neurological abnormalities and cognitive impairment that may be mistaken for metastatic disease. MRI shows patchy abnormalities within the white matter that enhance with gadolinium. Histologically, these patches contain an intense inflammatory infiltration with demyelination but axonal sparing. It is thought that the levamisole disrupts the blood-brain barrier potentiating 5-FU’s access to the CNS. Although treatment with steroids has been advocated, this syndrome is frequently self-limiting and patients usually recover completely over the course of several weeks without specific therapy. In some patients, thymidine has also been used successfully.58 Rarely, a peripheral sensory neuropathy has also been reported.65 Because many patients received adjuvant 5-FU based treatments for a variety of cancers, including breast, gastrointestinal, and bowel cancers, patients should be monitored for possible long-term neurocognitive damage.5
ASPARAGINASE
Asparaginase (either as the L- or pegylated formulation) is a component of remission-induction therapy used to treat acute lymphoblastic leukemia (ALL). It may cause cerebrovascular accidents during the first few weeks of its administration.66 This is due to depletion of plasma proteins involved in coagulation, such as fibrinogen and antithrombin III. Thrombosis typically occurs within the dural sinuses and cerebral veins, leading to secondary hemorrhage or infarction.67 Patients present with a range of symptoms from mild headache to coma and death, which are caused by rapid increases in intracranial pressure. Once diagnosed, the asparaginase should be stopped and the patient anticoagulated unless hemorrhage is present.
Asparaginase acts to cleave asparagine, an essential amino acid required by rapidly proliferating cells (hence its antimitotic action) and also as a neurotransmitter. Therefore depletion of asparagine during treatment has also been associated with the development of neuropsychiatric symptoms such as depression and hallucinations.68
CYCLOPHOSPHAMIDE
Cyclophosphamide is a prodrug, which alkylates DNA after hepatic activation.69 It is used in a wide range of malignancies, including lymphoma, breast cancer, and testicular cancer. When given in high doses, it may lead to inappropriate secretion of ADH (SIADH), and hence a secondary metabolic encephalopathy may occur, with confusion, seizures, or coma.70–72 There have been a few case reports of cyclophosphamide also being associated with blurred vision, dizziness, and confusion in the absence of SIADH.73,74
CYTARABINE
Cytarabine is an analogue of adenosine, causing chain termination during DNA synthesis; it is one of the most effective cytotoxic drugs in the treatment of acute leukemia. It is used either at a conventional dose of 100 to 200 mg/m2/day or at high doses of 2 to 6 g/m2/day.69 Neurotoxicity is particularly common at the higher dose levels, affecting 16% to 50% of patients;8 it predominantly affects the central nervous system. The risk of neurotoxicity is increased by age, dose/ schedule (particularly cumulative dose), renal or hepatic impairment, and the concurrent use of neurotropic antiemetics such as phenothiazines.8,75,76
The mechanism of toxicity is not well-understood, but it appears that cytarabine directly causes neuronal death, possibly by the inhibition of cytidine-dependent neurotropic signal transduction,77 although it has also been shown to stimulate the production of reactive oxygen species that may also damage DNA directly by inducing strand breaks.78
Acute cerebellar dysfunction is the commonest central neurotoxicity, occurring in approximately 14% of patients; they typically present with dysarthria, nystagmus, gait ataxia, and confusion. Onset usually occurs during administration of a multi-day regimen, particularly above a cumulative dose of 36 g/m2 and generally resolves rapidly once cytarabine is withdrawn.8,9,14 However, toxicity may be permanent once more than 8% to 20% of Purkinje cells have been lost.8 Acute encephalopathy is also common, presenting with somnolence or seizures. MRI scanning reveals diffuse high-intensity lesions within the central matter on T2-weighting that may be reversible.18 The encephalopathy should rapidly resolve entirely on stopping cytarabine; however, damage may be permanent and progress to leukoencephalopathy in a minority of patients, usually those with preexisting organ dysfunction or neurological problems.8 Less commonly, optic neuropathy, anosmia, and an incompletely reversible myelopathy have been reported.14 As with methotrexate, the intrathecal administration of cytarabine may cause ascending myelitis.8,79–81 There have also been case reports of sensory peripheral neuropathy following cytarabine exposure.82
ETOPOSIDE
Etoposide, a topoisomerase II inhibitor used in treatment of hematological, lung, ovarian, and testicular cancers,69 causes very little neurotoxicity, although at very high doses there have been reports of peripheral neuropathy, headache, seizures, and somnolence, in bone marrow transplant recipients and patients with malignant gliomas.83,84
FLUDARABINE
Neurotoxicity with the antimetabolite fludarabine is uncommon, but somnolence, acute encephalopathy, and chronic leukoencephalopathy progressing to coma and death have all been reported.85
IFOSFAMIDE
Central nervous system toxicity occurs in approximately 10% to 20% of patients receiving ifosfamide, who present with personality changes, confusion, hallucinations, stupor, and coma.9 More unusually, patients may experience seizures, myoclonus, cranial nerve palsies, or extrapyramidal symptoms.86,87 Symptoms usually begin 12 to14 hours after initiation of an ifosfamide infusion and spontaneously resolve 2 to 3 days after its cessation, although rarely symptoms may persist or even be fatal. EEG tends to show severe slowing with delta wave activity with or without seizure activity. Patients most at risk are those with impaired renal function, low serum albumin, pelvic tumors, and previous exposure to cisplatin.88 The risk of encephalopathy varies with route of administration. It is more common after oral administration, and is also more frequent with short intravenous infusion durations.89–91 The mechanism of toxicity is unclear but may be related to accumulation of metabolites such as chloracetaldehyde and chloro- ethylamine, which deplete intracellular glutathione and NAD and impair mitochondrial electron transport.92 Methylene blue, 300 mg IV in 6 divided doses, is used in the treatment of ifosfamide-induced encephalopathy and 50 mg IV qds may be given prophylatically.93–96 It is thought to act primarily as an alternative electron acceptor restoring mitochondrial respiratory chain function, but may also oxidate NADH and inhibits plasma monoamine oxidases.
METHOTREXATE
Methotrexate is one of the most widely used cytotoxic antimetabolites in the treatment of hematological, breast, and head/ neck cancers.9 In addition, prophylactic intrathecal use in ALL and high grade non-Hodgkin lymphomas has reduced the incidence of CNS relapse in high risk patients.97 Methotrexate acts by inhibiting dihydrofolate reductase, thus blocking purine and thymidine biosynthesis. Its neurotoxicity is thought to stem from widespread disruption of various metabolic pathways in the brain and can be acute or chronic.
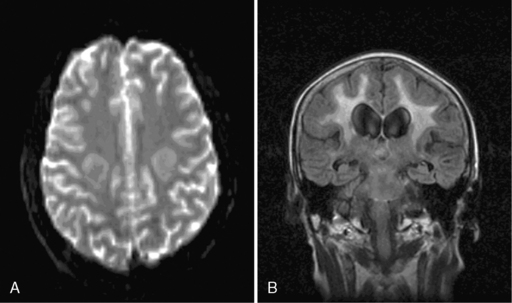
Acute encephalopathy is associated with the administration of high-dose methotrexate (>3 mg/m2) and is characterized by somnolence, confusion, and seizures.98 Other symptoms include emotional lability and alternating hemiparesis, giving rise to the misdiagnosis of a functional disorder. The imaging appearances are characteristic and show symmetrical restricted diffusion on diffusion-weighted imaging, even when the T2-weighted sequences appear normal (Figure 16-1). Patients most at risk are children, those who have received previous cranial radiotherapy, or those receiving concomitant intravenous and intrathecal therapy. Metabolic abnormalities associated with the development of encephalopathy include widespread reduction in glucose utilization and protein synthesis,99 which are reversible by replacing depleted folate stores with the administration of leucovorin.100 Leucovorin is now given routinely following high-dose methotrexate (folinic acid rescue). Other studies have also postulated that changes in adenosine,101 homocysteine,102,103 or biopterin104 levels may also contribute to development of encephalopathy. In one study, aminophylline (2.5 mg/kg IV over 1 hour), given to six encephalopathic patients, caused immediate resolution of symptoms in four and improved symptoms in the remaining two patients.101
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree