New Therapeutic Directions
Robert S. Fisher
Guy M. McKhann II
John M. Stern
Mark S. Quigg
Jean Régis
Donald L. Schomer
Steven C. Schachter
Annamaria Vezzani
Introduction
People with epilepsy, their families, and the clinicians who care for them await the advances that will cure or prevent epilepsy, but all want to know what can be done now (or in the near future) when medications fail or are unrelentingly toxic? This chapter collects a miscellaneous group of therapies that may partially address this question. By selection criteria to be in this chapter, none of the therapies yet is proven as safe and effective. Most, but not all, are available in experimental protocols, none as part of standard therapy.
A general theme of the experimental therapies in this chapter is an attempt to be relatively noninvasive. Surgical procedures should not have to remove more than the minimal amount of brain necessary to stop seizures, or perhaps they should remove no brain at all. Electrical brain stimulation therapies have attempted seizure control without resection. Several targets and strategies have been employed, but most active now are deep brain stimulation in the anterior or centromedian thalamus, subthalamus, or hippocampus, or direct stimulation of a cortical seizure focus. Stimulation can run on a cycle, as with vagus nerve stimulation for epilepsy. Alternatively, stimulation can be made contingent upon recording an electroencephalographic (EEG) pattern indicative of an ongoing (seizure recognition) or impending (seizure prediction) seizure. Noninvasive trans-cranial magnetic stimulation has been tested for seizure control, since transcranial electrical stimulation tends to be painful. Transcranial magnetic stimulation used for diagnostic purposes is discussed elsewhere in this volume.
Focal brain radiation may or may not be less invasive than surgery, but some investigators argue that it is. More important than the avoidance of a craniotomy with Gamma Knife or CyberKnife radiation therapy is the possibility that radiation can spare cells with a sublethal dose but still inhibit seizures.
Why distribute an antiepileptic drug to every region of brain and body when it might only be needed within a restricted region of the central nervous system (CNS)? Why should a kidney stone or severe leukopenia be a risk of therapy for treating the brain? Techniques to distribute medication focally in the brain include intracerebroventricular perfusion, catheter-mediated perfusion in the cortex, drug-eluting polymer wafers, targeted liposomes, seizure-activated prodrugs, and cell transplants that release drugs. The genetic machinery of the neurons and glia in a seizure focus can be altered by gene transplants via viral vectors. Altered genes can produce renewable local neurotransmitters or neuromodulators that serve to inhibit local excitability.
Not every exciting potential new therapy can be covered in one chapter. For example, rapid cooling of a seizure focus113 has been shown to be effective in blocking seizures in animal systems and in small uncontrolled series of patients. In the following section, investigators working with some of the new technologies will provide a very brief overview of the methods, promise so far, and potential limitations. The hope is that some of these therapies will prove successful, and migrate to the standard care sections of the future editions of this compendium.
Deep Brain Stimulation
Electrical brain stimulation to control intractable epilepsy has been attempted intermittently for several decades, using a variety of neural targets: Cerebellar cortex, deep cerebellar nuclei, caudate nucleus, locus ceruleus, hippocampus, centromedian nucleus of thalamus, anterior nucleus of thalamus, subthalamic nucleus, neocortex, and vagus. Space limitations do not permit review of each stimulation site, but reference may be made to several published recent reviews.36,40,45,55,65,67,79,82,134 Stimulation of brain tissue to control or partially control medically intractable seizures has several potential advantages over resective surgery. Firstly, stimulation may influence a seizure focus or multiple foci in regions of brain, for which it would not be safe to remove tissue. Secondly, electrical stimulation is an adjustable therapy, with many parameters available for individualization on an empirical basis for each patient. Thirdly, stimulation can be discontinued, as opposed to an irreversible surgery. Nevertheless, clinical experience and at least one randomized study148 overwhelmingly support resective surgery in circumstances for which a seizure focus safely can be removed. Neural stimulation, therefore, is a candidate for a palliative role, in circumstances for which seizure control is not possible with medications or removal of the seizure focus.
Stimulation of the thalamus for epilepsy originally was proposed by the pioneering neurosurgeon Irving Cooper.17,18,19,143,144 Only a few studies so far have investigated basic mechanisms of electrical stimulation for seizures. The results of stimulating neural tissue are not predictable from basic physics and electrical field principles. Complications include a complex geometry of nuclei and tracts in the brain; “sign-changing” circuitry, by which inhibitory pathways may be activated or deactivated; exquisite dependence of outcome on parameters of electrical stimulation, such as frequency, intensity, bipolar versus referential application, and intermittent or continuous stimulation; and many other parameters, including some that undoubtedly are not yet recognized. Studies with in vitro slice tissue indicate that both alternating currents73 and direct-current electrical fields108 can in some circumstances inhibit epileptiform activity in the model systems. One likely mechanism may be the polarization of neurons with release of extracellular potassium, leading to an induced spreading depression.8 Inactivation of sodium channels from excess depolarization, thereby blocking action potential formation, may play a role. Direct effects on inhibitory or excitatory systems by stimulation also could reduce excitability in the brain.
Stimulation of the anterior region of the thalamus in rats can raise the threshold for seizures provoked by the acute application of the convulsant drugs pentylenetetrazol90 and pilocarpine.44 However, a recent study69 failed to find benefit, and in fact observed increased seizure frequency, with anterior thalamic stimulation in rats with chronic epilepsy induced by previous doses of kainic acid.
In the 1990s, electrical stimulation to treat epilepsy rarely was the object of serious study, until two events refocused interest on this potential new modality: Success of vagus nerve stimulation for epilepsy7 and success of deep brain subthalamic stimulation for movement disorders.4 Two types of brain stimulation currently are in multicenter pivotal clinical trials for treatment of epilepsy: Open-loop stimulation and closed-loop stimulation, which also is referred to as responsive or contingent stimulation. Open-loop stimulation delivers stimuli on a scheduled basis, either intermittently or continuously, but not dependent upon detection of epileptiform activity. Responsive neural stimulation (see elsewhere in this chapter) uses EEG sensors to detect epileptic stimulation and deliver stimuli to the region of the suspected seizure focus or foci. Open-loop electrical stimulation of human diencephalon has been performed in the centromedian thalamus, subthalamus, and anterior thalamic nuclear group.
Centromedian Stimulation
Velasco et al.146 identified the centromedian nucleus of thalamus as a promising target for electrical stimulation. To date, this target represents the most stimulated site in the brain for epilepsy, but no large, controlled trial has yet been completed of centromedian stimulation to document efficacy. The largest group of patients with centromedian stimulation was published by Velasco et al.145 A total of 49 patients with a variety of intractable seizures were subjected to bilateral centromedian stimulation at intensities of 2.5 to 5 V, with stimulation frequencies ranging from 60 to 130 Hz. In the study population, centromedian stimulation was effective against generalized tonic–clonic seizures, tonic seizures, and atypical absence seizures, but not against complex partial seizures. A small cross-over trial of centromedian stimulation in seven patients with mixed seizure types29 showed a trend toward benefit, but not of statistical significance.
Subthalamic Nucleus Stimulation
The subthalamic nucleus is a primary target for electrical stimulation to ameliorate tremor and Parkinson symptoms.4 Laboratory studies suggest that subthalamic stimulation also can benefit seizures in animal models of epilepsy produced by kindling,70 genetic absence,147 fluorothyl,70 and kainic acid.79 Nine patients treated with subthalamic stimulation for epilepsy have so far been reported.5,13,21,79 Six of the nine were said to show more than a 75% improvement in seizure frequency, although each of these reports was uncontrolled.
Anterior Thalamic Stimulation
The anterior thalamic nucleus provides efferents to superior medial frontal and cingulate cortex. In turn, the cingulum white matter projects to the entorhinal cortex, and thereby to the hippocampus. Stimulation of the anterior thalamic nucleus therefore is well placed to influence certain frontal and temporal seizures. Initial work on thalamic stimulation for epilepsy was performed by Cooper, with published results on several dozen patients.19,143 These studies reported beneficial outcome in several patients, but details and long-term follow-up results were not provided in the publications. Sussman et al.128 reported in abstract form the benefit of anterior thalamic stimulation at 100 Hz and approximately 5 V in three of five patients tested. Subsequently, pilot studies of anterior nucleus thalamic stimulation were performed on 14 patients from four different study sites.40,48,58
Table 1 Responsive Stimulation after Induced Seizures | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||
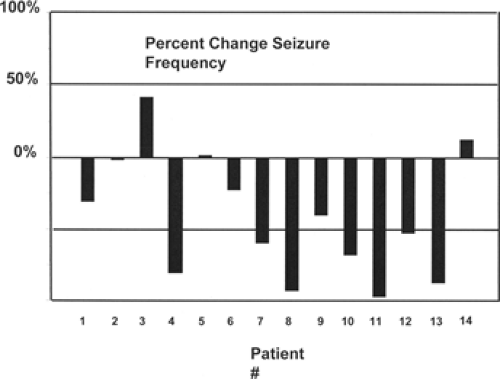
Figure 1 shows the changes in seizure frequency for the patient group before and after stimulation. The mean seizure frequency at 12 months of stimulation was 55% ± 39% of baseline, and 57% of patients exhibited at least a 50% improvement in seizure frequency (responders). Among those believed to have frontal or temporal seizure foci, the responder rate was 67%.40 Five patients had seizures capable of producing falls; four of these had a marked reduction in such seizures.58 None of the 14 patients in the pilot trial experienced a serious complication. However, placement of deep brain stimulation electrodes is known to comprise a measurable risk for hemorrhage9 and infection.132 One patient implanted at a fifth institution using a similar thalamic stimulation pilot protocol had a hemorrhage leading to contralateral hemiparesis. Hodaie et al. in Toronto48 have suggested that implantation itself might account for much of the perceived benefit, either by a microlesion effect or placebo effect.
Based on anecdotal and uncontrolled evidence of possible efficacy for anterior thalamic stimulation, a pivotal, multicenter, randomized, and blinded trial has been launched, called SANTE, for Stimulation of the Anterior Nucleus of Thalamus for Epilepsy (see www.epilepsycontrol.com). Eligible patients are those with medically uncontrolled partial or secondarily generalized seizures, not believed amenable to surgical resection. Seizures must be severe enough to interfere seriously with quality of life and must occur at a frequency of at least six per month. The SANTE trial utilizes bilateral implantation of deep brain-stimulating electrodes in the principal portion of the anterior nuclei, connected to a dual-channel Intercept (Medtronic) stimulation module. Stimulation parameters are 145 pulses per second with 90-μsec (approximately 0.1 msec) duration pulses, charge-balanced negative and positive on for 1 minute and off for 5 minutes. Voltage is set to 5 V in the treated group and 0 V in the placebo group. Since the patients, caregivers, or treating medical team cannot perceive the stimulation, this is a true double-blind study. After a 3-month baseline phase, patients receive either 5 V or 0 V stimulation for 3 months, then open-label therapy via a limited number of
parameter sets for 10 additional months. At time of this writing, the study had passed the midstudy “futility analysis” by the unblinded data-monitoring safety board, and the investigators have been given the go-ahead to complete the trial. Final outcome of the trial is not yet known.
parameter sets for 10 additional months. At time of this writing, the study had passed the midstudy “futility analysis” by the unblinded data-monitoring safety board, and the investigators have been given the go-ahead to complete the trial. Final outcome of the trial is not yet known.
Responsive Neurostimulation
Some patients with intractable epilepsy either are not candidates for surgery or do not want to undergo resective or disconnective surgery. For these patients, one option that is currently under investigation is responsive stimulation. Responsive stimulation, as currently available, differs from deep brain stimulation in two major ways: (a) deep brain stimulation involves continuous open-loop therapy delivered into a target region of interest. It is delivered all the time without detection of brain activity in the target or feedback from the target tissue. In contrast, responsive stimulation is closed loop. It is delivered intermittently in response to detected EEG abnormalities. (b) The pulse generator for available deep brain stimulation (DBS) systems is implanted in the chest wall below the clavicle, while the currently available responsive stimulator is implanted entirely within the skull.
The goal of responsive stimulation is to provide brief target stimulation only in response to an EEG-detected “preictal state” or ictal onset. For this approach to work successfully, it requires a precise methodology to detect ictal or preictal EEG activity on the afferent side of the loop and effective stimulation delivery on the efferent side of the closed loop.
The Afferent Loop: Seizure Detection
In order to terminate clinical seizures by responsive stimulation, the seizure must be detected and treated before it spreads to a degree that it cannot be controlled with focal stimulation. This suggests that this therapy will be most effective if stimulation is delivered as early and precisely in the epileptic discharge as possible, usually before the onset of clinical symptoms. Responsive stimulation thus requires sufficiently accurate seizure prediction algorithms (SPAs) for EEGs recorded from intracranial electrodes.
All recent SPAs utilize a “sliding window analysis” in which a window of recorded EEG activity is mathematically analyzed using either linear or nonlinear algorithms. Linear algorithms calculate particular features directly from the EEG, including autocorrelation, spectral band analysis, curve length, accumulated energy, and high-frequency epileptiform oscillation78,92 nonlinear algorithms. The EEG sliding window is reconstructed in three-dimensional phase space and analyzed using techniques such as the short-term maximum Lyapunov exponent, dynamic similarity, or correlation dimension.78,92 All of these techniques have been purported to have their advantages in seizure prediction. However, as discussed at the recent Second Annual Seizure Prediction Workshop in April 2006,77 there is still much work to be done in terms of understanding and applying the basics of seizure dynamics. Future techniques will likely apply multichannel, multialgorithm integrated analysis, utilizing a continuous probability curve rather than binary thresholding. In addition, the relevance of high-frequency epileptiform oscillations prior to seizure onset will need to be determined and incorporated into future SPAs. It is likely that future seizure prediction and detection in an individual patient will utilize more than one SPA, and that the optimal SPA application profile will differ from patient to patient.
The Efferent Arm: Stimulation Delivery
In humans, cortical stimulation has long been known to abort spontaneous or induced epileptiform activity during brain mapping in surgical patients. Penfield and Jasper first applied focal electrical stimulation over 50 years ago to terminate spontaneous seizures detected by electrocorticography (ECoG) at the time of resective surgery. More recently, stimulation has been used to shorten or terminate epileptiform afterdischarge activity in patients implanted with intracranial subdural electrode arrays. During the brain-mapping procedures in these implanted patients, brief electrical stimulations were applied to the cortex, with the stimulus amplitude progressively increased until a clinical alteration or subclinical electrographic afterdischarge resulted. In 17 patients with subdural electrodes who underwent functional mapping, control afterdischarge duration was compared with afterdischarges that received brief bursts of stimulation.72 A biphasic 50-Hz pulse was presented for 0.3 to 2 seconds at the same stimulus intensity used to generate the afterdischarge. The duration of the afterdischarges was significantly shorter in those patients receiving stimulation (Table 1).
Brief pulse durations (0.5 to 1 second) were more effective in terminating afterdischarges than were stimulations of longer duration. This and other studies suggest that the exact stimulation parameters and timing may be very important in determining whether stimulation will disrupt or further entrain electrographic discharges.
Closing the Loop: Combined Detection and Delivery Systems
Limited animal model and human experience pertains to responsive stimulation. In the genetic absence epilepsy rat of Strasbourg (GAERS) rat, which has a genetic disposition to spontaneous generalized absence seizures, responsive stimulation delivered to the subthalamic nucleus upon detection of
spike-and-wave epileptiform activity was effective in suppressing seizures.147 Interestingly, continuous stimulation had no effect on seizure frequency in this model. Responsive stimulation has also suppressed spontaneous seizures in cats100 and in rat hippocampal slices.94
spike-and-wave epileptiform activity was effective in suppressing seizures.147 Interestingly, continuous stimulation had no effect on seizure frequency in this model. Responsive stimulation has also suppressed spontaneous seizures in cats100 and in rat hippocampal slices.94
While still in its infancy, the field of responsive stimulation already has examples of effective clinical application in human epilepsy patients. Osorio et al.97 at the University of Kansas applied high-frequency electrical stimulation that was delivered either directly to the epileptogenic zone (local closed loop, n = four patients) or to the bilateral anterior thalamic nuclei (remote closed loop, n = four patients) in response to every other automated seizure detection. The eight patients had a baseline video-EEG monitoring with implanted subdural and depth electrodes that localized epileptic foci and quantified seizure frequency using a linear simple genetic algorithm (SGA). Patients determined to have multifocal onsets were implanted into the bilateral thalami for remote stimulation, while local therapy was delivered to patients with a precisely localized epileptogenic focus. A total of 1,491 stimulations were delivered, 0.2% of which triggered afterdischarges. The mean reduction in seizure rate in the local closed-loop group was 55%; in the three responders the mean decrease was 86%, with two patients rendered seizure free during the local stimulation therapy. In the remote thalamic closed-loop stimulation, the mean seizure reduction rate was 41%, with a 74% reduction in the two responders.
More recently, patients undergoing subdural and depth electrode monitoring for seizure localization and functional mapping were enrolled in a trial testing an externalized version of an implantable responsive neurostimulator (eRNS, NeuroPace, Inc., Mountain View, CA).64 This device used linear SPAs that could be tuned to patient-specific epileptiform activity to deliver electrical stimulation through up to eight contacts of a combination of subdural and/or depth electrodes implanted at the epileptogenic zone. Of 50 enrolled patients, 40 received responsive stimulation. The experience with four of these patients was subsequently reported. In this trial, electrographic seizures were altered and suppressed in these patients during trials of neurostimulation, with no major side effects. In one patient, stimulation appeared also to improve the baseline EEG.
Implantation of an internalized version of this responsive neurostimulator (RNS) system was subsequently investigated in a multicenter clinical trial assessing feasibility of the device’s clinical implantation and implementation. For this trial, enrollment included subjects 18 to 65 years with intractable partial-onset seizures and localized epileptogenic-onset region or regions. Subjects with at least 12 simple partial (SP) sensory or motor, complex partial (CP), or generalized tonic–clonic (GTC) seizures over an 84-day baseline period qualified for implant. The responsive stimulator was connected to up to four contact leads (subdural and/or depth), which were targeted to the seizure focus. This trial’s results have been reported in limited fashion. A single center’s experience with eight implants resulted in 45% reduction in seizure frequency in seven of eight patients over more than 9 months’ follow-up.30 For the multicenter trial, efficacy was assessed during the most recent 84 days for which a subject could have received therapy. Defining response as at least a 50% reduction in seizures, the responder rate for 56 subjects was 36% for CP, 50% for GTC, and 36% for totally disabling (TD) seizures. The median percentage reduction was CP 28%, GTC 50%, and TD 30%; seizure reduction was significant for CP (p <0.005), GTC (p <0.02), and TD (p <0.001). In 65 implanted subjects, including 17 device replacements, there were no serious unanticipated device-related adverse events. Responsive neurostimulation was well tolerated by the patients.37
 FIGURE 2. Schematic view of the implantable NeuroPace responsive stimulation device (NeuroPace, Inc., Mountain View, CA). |
In follow-up to the feasibility trial, a clinical efficacy trial began enrollment in the fall of 2006. In contrast to available implantable deep brain stimulation systems, in which the pulse generator is implanted below the clavicle, the RNS system has two four-contact subdural strip and/or depth electrodes that are attached to a seizure detection and stimulation delivery device that is implanted directly into the skull (Fig. 2). In our center’s experience, the most commonly encountered current applications for the RNS trial are dominant mesial temporal seizure onsets in patients with preserved hippocampal function, bitemporal epilepsy with mesial temporal onsets, and seizure onsets arising from a functionally “eloquent” area such as essential language cortex.
Future Directions
Responsive stimulation therapy for epilepsy must be efficacious, well tolerated, and safe in order to offer an alternative to resective surgery or other stimulation modalities in patients resistant to antiepileptic medication. Therapy should be targeted at the epileptogenic region or at seizure propagation pathways and leave other regions of the brain unaffected. It need not interfere with normal brain function, have an acceptable false-positive detection rate, and be stable as a therapy over time. It must also not result in chronic brain damage or the generation of new epileptic foci.
In addition to responsive stimulation, a device with accurate SPA technology will likely be paired in the future with novel treatment strategies such as convection-enhanced local drug delivery126 or focal cooling113 to treat epilepsy in closed-loop responsive fashion. Future therapy may be delivered to the epileptic focus, to the epileptogenic region, to propagation pathways, or to deep brain structures. Whether a local responsive treatment can both suppress seizures and inhibit epileptogenesis remains to be determined.
As discussed above, SPA technology will certainly improve and need to be optimized on a case-by-case basis. The ability of responsive stimulation to then terminate detected seizures may depend on a variety of stimulation parameters including exact timing of delivery, current intensity, stimulus duration, the stimulus waveform, pulse frequency, and the delivery of the
stimulation in relation to the morphology of the epileptiform activity. These are all areas requiring further basic and human investigation.
stimulation in relation to the morphology of the epileptiform activity. These are all areas requiring further basic and human investigation.
The exact localization of the epileptogenic zone is critical for the future efficacy of responsive therapies. Currently, many mesial temporal and nearly all neocortical responsive stimulation patients require invasive monitoring to determine their seizure onset location or locations. Advances in techniques such as magnetic resonance imaging (MRI)–based localization of interictal and ictal activity have great promise in determining where to apply responsive treatment.
Transcranial Magnetic Stimulation
Transcranial magnetic stimulation (TMS) is a relatively new technique in neurophysiology, with its first description only approximately 20 years ago.3,62 In contrast to the preexisting transcranial electrical stimulation, TMS provides noninvasive cerebral stimulation that is painless.89 TMS achieves this by using electromagnetic induction to produce the neurostimulation. The induction is produced by placing a rapidly changing magnetic field adjacent to the scalp. The field is generated by a brief but large electric current through a wire loop and produces an electric current in the subjacent cerebral cortex. This results in synaptic excitation of the neurons within the region of the electric current. Because the magnetic field penetrates the tissue with negligible resistance or attenuation, it is painless. Each magnetic pulse is perceived as a touch or a tap to the scalp.
Technique
TMS has the potential for excellent spatial and temporal resolution. A 0.1-msec stimulation induces a current lasting the same duration and confined to 1 cm2 of cortex with a maximum depth of about 2 cm.57,112 When the motor cortex is stimulated, the excitation affects corticocortical neurons predominantly with subsequent activation of corticospinal neurons. The produced movement is a motor-evoked potential (MEP) with measurable conduction time and amplitude. Beyond the stimulation of motor cortex, all cortex that is adjacent to the cranium may be stimulated. Depending on the stimulation parameters, TMS may produce transient dysfunction for purposes of functional mapping or may temporarily alter the intrinsic excitability of the local gray matter. It is this capacity to alter the intrinsic excitability of cerebral tissue that is the foundation of TMS as a potential treatment for epilepsy.
Individual TMS pulses provide a means to measure cortical excitability through several parameters, including intensity thresholds, post-MEP (central) silent periods, and paired-pulse techniques that are similar to those used in basic neurophysiology.131 These techniques assess different aspects of cortical excitability. Threshold studies apparently represent pyramidal cell membrane excitability as the results are affected by sodium and calcium channel–blocking medications. Central silent period studies likely represent intracortical inhibition and paired-pulse studies are influenced by networks beyond the local cortex. The results of both are dependent on glutamatergic, dopaminergic, and GABAergic (γ-aminobutyric acid) activity.
Single and paired-pulse stimulations do not produce a sustained alteration to the cortical excitability, but such alterations may occur when the stimulation is delivered in trains of pulses. This technique is called repetitive TMS (rTMS). Low-frequency rTMS (LF-rTMS) is defined as pulse trains at or below 1 Hz and high-frequency rTMS (HF-rTMS) as pulse trains above 1 Hz and usually around 20 Hz. The 1-Hz division has practical implications because LF-rTMS is associated with decreases in cortical excitability and HF-rTMS is associated with increases. The occurrence of seizures as an adverse effect of TMS most often occurs during HF-rTMS. Therefore, LF-rTMS is the technique that has been most explored as a treatment for epilepsy. This approach partly depends on abnormal excitability of epileptic cortex, as documented in clinical and animal studies. LF-rTMS delays the development of seizures in pentylenetetrazol-exposed and kindled rats.1
Clinical Experience for Epilepsy Therapy
In humans, the reports of rTMS efficacy for seizures have varied considerably with respect to the types of patients included and outcome. Direct electrical stimulation through subdural grid electrodes can decrease both interictal epileptiform discharges and electrographic seizures in patients.60,116 In a case report of a patient with a focal cortical dysplasia, 100 pulses of LF-rTMS were delivered to the area over the dysplasia twice a week for 4 weeks with a resulting 70% reduction in seizure frequency and a 77% reduction in interictal epileptiform discharges.88 A report of two patients with epilepsia partialis continua describes each receiving 15 minutes of intermittent HF-rTMS and one becoming seizure free for 2 weeks. Both patients demonstrated a decrease in seizure-related hyperperfusion on single photon emission computed tomography (SPECT).39
A series of five patients with intractable focal epilepsy received LF-rTMS over 3 months with modest efficacy.11 Collective reduction in seizure frequency was 23%, but only one individual within the group demonstrated a significant reduction, which was 43%. The others showed no significant change in mean daily seizure number. In a series of nine patients with various types of focal epilepsy, 1,000 pulses of LF-rTMS were delivered to the vertex daily for 5 days. This produced a seizure frequency reduction >50% for three patients, a 20% to 50% reduction for three patients, a 20% reduction for one patient, and no improvement for the remaining two patients.133 Those who benefited continued at the reduced frequency for 6 to 8 weeks. A series of seven patients with extratemporal lobe epilepsy received stimulation with 810 pulses of LF-rTMS daily for 5 days.59 The average frequency of complex partial seizures decreased by 36%, but the decrease in total seizures did not reach statistical significance.
Theodore et al. delivered 30 minutes of LF-rTMS each day for a week to the scalp over the identified epileptic focus in a series of 24 patients.135 Using a controlled, blinded study design, they observed a 36% reduction in seizure frequency for those patients with neocortical epilepsy and a 12% reduction for those with mesial temporal lobe epilepsy, but the difference was not statistically significant. A similarly designed study with baseline, intervention, and follow-up periods included four patients and observed a seizure frequency reduction >50% in three patients.115 This study differed from the prior by focusing the stimulation with frameless stereotaxy at a well-defined epileptogenic region, which may explain its more uniform results.
Conclusion
Overall, rTMS has demonstrated promise as a treatment for some forms of epilepsy. The rapid decrement of magnetic field strength with distance suggests that rTMS might be most useful with superficial epileptogenic regions, such as with cortical dysplasias. Further development of rTMS as a treatment will require controlled studies that are designed to determine efficacy and identify patient characteristics that are most associated
with successful treatment. Technical development of rTMS should establish optimal stimulation parameters for affecting seizure frequency by addressing the questions of stimulation intensity, frequency, duration, and targeting. If future clinical investigations identify rTMS methods that produce consistently superior seizure control results, epilepsy care would have the additional benefit of gaining a safe and highly tolerable treatment.
with successful treatment. Technical development of rTMS should establish optimal stimulation parameters for affecting seizure frequency by addressing the questions of stimulation intensity, frequency, duration, and targeting. If future clinical investigations identify rTMS methods that produce consistently superior seizure control results, epilepsy care would have the additional benefit of gaining a safe and highly tolerable treatment.
Table 2 Remission of Seizures Following Radiosurgery of Arteriovenous Malformations | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
Gamma Knife Radiosurgery
Gamma Knife radiosurgery (GKS) allows performance of precise radiosurgical lesions deep or adjacent to eloquent cortex or other regions of the nervous system that cannot be accessed easily by conventional surgical approaches. In addition, its ability to target functional lesions noninvasively may be advantageous for select patients. On the other hand, use of GKS requires precise localization of the epileptogenic brain region, which means that its noninvasive advantage becomes somewhat moot in syndromes that require invasive localizing techniques.
Relevant background on Gamma Knife history and methodology can be found in the cited reviews.33,71 Briefly, the Gamma Knife stereotactically projects multiple gamma radiation sources onto brain lesions (Fig. 3). Whereas the individual sources of radiation are too weak to damage intervening brain tissue, a lesion within the common focus is exposed to high-intensity radiation. It is not known how irradiation produces an antiepileptic effect. Massive necrosis of neuronal tissue does not seem to be a requirement, as the major pathologic findings following irradiation are endothelial damage to small blood vessels and astrocytic reactions.150 One hypothesis, therefore, is that neuronal damage results from ischemia caused by vascular inflammation.120 In addition, some neurons (e.g., hippocampal neurons) may show differential susceptibility to irradiation or ischemic injury.140
The main use of GKS is in the treatment of tumors117 or arteriovenous malformations (AVMs)35,47,66,127 that are inoperable with standard neurosurgical techniques. In patients with tumors, it is difficult to separate the effects of GKS as an antiepileptic procedure from its beneficial effects on the primary lesion. The potential efficacy of GKS in the treatment of symptomatic localization-related epilepsies is most evident in the treatment of AVMs. Steiner et al. reported that 69% of patients with recurring seizures due to an AVM became seizure free after GKS.127 Nineteen percent successfully discontinued all anticonvulsant medications. Subsequent studies of both proton beam and GKS showed a combined rate of 70% seizure remission following Gamma Knife treatment of AVMs (Table 2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree