9 Non-Invasive Strength Analysis of the Spine Using Clinical CT Scans
KEY POINTS
Introduction
Osteoporosis is widely recognized as an underdiagnosed and undertreated disease. According to the National Osteoporosis Foundation and the National Institutes of Health, 10 million Americans are estimated to have osteoporosis, and another 34 million are at increased risk due to low bone mass, but only about 20% of those eligible to be screened are actually tested and only a fraction of those are positively diagnosed and treated. Above age 50, the density of vertebral trabecular bone decreases at a rate of about 2.2% to 3.0% per year for women, depending on age, and by about 1.7% to 2.5% per year for men,1 with about 700,000 osteoporotic spine fractures occurring annually in the united States2.
Management of osteoporosis in the over-50 age group is important both to avoid such fractures and to optimize spine surgery outcomes. A recent study from Taiwan3 estimated that for all major spine surgical cases, not including vertebroplasty or kyphoplasty, 47% of women and 46% of men over age 50 had low bone mass or “osteopenia” — a BMD T-score of between −1.0 and −2.5 — and 44% of women and 12% of men had osteoporosis — a BMD T-score of less than −2.5. As the size of the aging population continues to increase, a huge and growing proportion of spine surgery patients may have compromised bone strength. This presents a challenge to the spine surgeon using any sort of instrumentation or implant for stabilization, since the underlying bone and the bone-implant interface need to be strong enough to sustain the stresses both from daily activities and spurious overloads.
A number of different types of imaging modalities are now available for noninvasive assessment of bone density, structure, and strength.4 The dual-energy x-ray (DXA) scan is the current clinical standard for bone density assessment. However, DXA for the spine has a number of limitations. Being a 2D imaging modality, a DXA scan combines all bone morphology in the anterior-posterior direction. Thus, arthritic changes in the posterior elements, degenerative osteophytic growths around the endplates, and aortic calcification all produce bone mineral density increases in the DXA scan — increases that confound the measurement of bone mineral density in the load-bearing vertebral body. DXA scans also provide very limited information on the morphology, density, or strength of the pedicles. As a result of these limitations, DXA of the spine is less predictive of the risk of osteoporotic fractures than is DXA of the hip, DXA of the spine can be highly misleading in terms of measuring actual bone mineral density of the vertebrae or pedicles, and there remains a need for improved strength and fracture risk assessment of the spine.
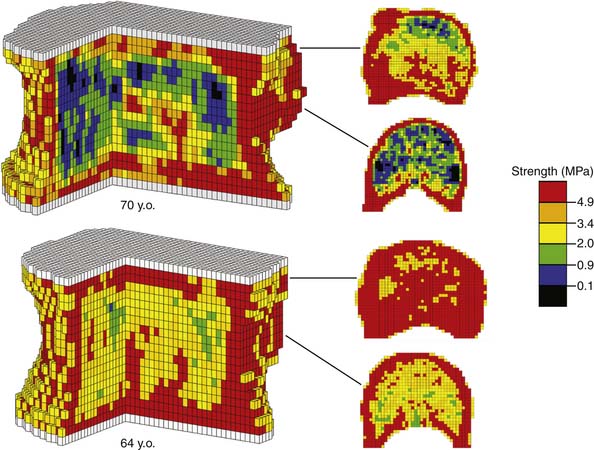
Computed tomography (CT), being a 3D imaging modality, provides a powerful alternative to DXA and is preferable to magnetic resonance imaging (MRI) for bone strength assessment since it provides quantitative information on bone mineral density.4 One limitation with CT analysis is the difficulty of interpreting the large amount of information in the scan in terms of a clinically relevant outcome such as bone strength. This is because a low value of bone mineral density at a particular location within the bone does not necessarily indicate a problem with overall bone strength. Conversely, such a local decrease in density may not show up in an averaged measure of bone mineral density, but may be problematic if that local decrease in density occurs in such a location as to appreciably compromise strength. To overcome this limitation, a sophisticated engineering structural computational analysis technique known as “finite element analysis” can be applied to CT scans to provide an estimate of vertebral strength,5 in much the same way as engineers perform computational strength analysis of such complex 3D structures as bridges, aircraft components, and engine parts (Figure 9-1). The resulting “biomechanical computed tomography” (BCT) technology, which represents a post hoc analysis of a clinical CT exam, is now being used in a variety of clinical research studies that address vertebral strength, aging, osteoporosis and its various therapeutic treatments. Because BCT creates a mechanical model of the patient’s bone, it can also be adapted to include a virtual implant and in that way provide estimates of strength and stability of various bone-implant constructs — all from analysis of a patient’s preoperative CT scan.
Clinical Case
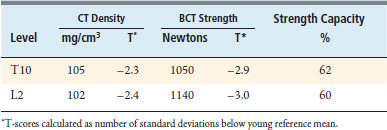
The BCT analysis was used to estimate the vertebral strength for T10 and L2 in order to better assess the osteoporotic status of the vertebrae (Table 9-1). Analysis of the scans showed substantial posterior arthritic changes and that the bone strength was three standard deviations lower than the mean value for a young reference population. The volumetric density scores of the trabecular bone based on the CT data indicated low trabecular bone density — almost in the osteoporosis range — but they did not reflect that this patient had low cortical density and relatively small bones, both of which also contributed to her very low bone strength. The DXA spinal T-scores were therefore misleading because of the substantial posterior calcification, arthritic changes, low cortical density, and small bone size. Calculations of the strength-capacity — which take into consideration the expected magnitude of the in vivo forces acting on the patient’s spine (see later in the chapter for more details) — were in the 60% range, indicating that the strength of this patient’s vertebra was only about 60% of what it should be in order to safely lift a 10-kg object with back bent (a “worst case” strenuous loading condition). Based on these findings, the surgeon instrumented from T12-L1, advised the patient of her elevated risk of vertebral fracture, and referred her for an endocrine consultation.
Basic Science
Aging of the Spine
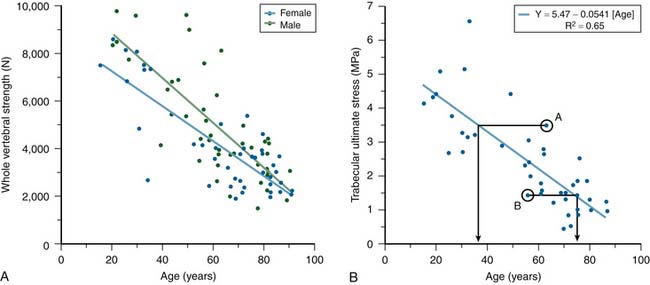
Substantial changes occur to vertebrae with aging. Cadaver studies have shown that whole vertebral strength decreases by about 12% per decade from ages 25 to 85 (Figure 9-2). Although these changes are due primarily to a loss of bone density, which is offset in part by subtle increases in bone size, the loss of cortical bone is generally not as pronounced as the loss of the trabecular bone.1 DXA generally is unable to distinguish between cortical and trabecular bone in the spine, due to its projectional nature. Aging of the spine is also accompanied by osteoarthritic changes (formation of osteophytes, etc.) around the disc and endplates. Again, due to projectional limitations, such degenerative changes are manifested as increases in BMD on DXA exams — effectively adding noise to the BMD signal from the more biomechanically relevant vertebral body portion of the spine. There is also substantial heterogeneity in trabecular strength across the population at any age (Figure 9-2). Thus, although advanced age is associated with low bone strength, age, sex, and DXA information are inadequate for clinical assessment of vertebral strength for an individual patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree