Figure 51.1. Non-contrast enhanced head CT obtained in a patient with SAH.
- Technique. The scan plane should be parallel to the hard palate. It should be noted that the sensitivity of CT depends directly on the amount of blood extravasated into the subarachnoid space. If there are only small amounts of bleeding, the sensitivity of CT can be increased by cutting thin slices (3 mm) from the base of the skull.
- Time since the onset of symptoms. In the first 12 hours, CT can reveal extravasation of blood into the subarachnoid space in approximately 100% of cases; however, this percentage decreases to 93% between 12 to 24 hours. Over the following days, the blood circulation and elimination from the subarachnoid spaces causes the sensitivity of CT to decrease markedly. It is estimated that the sensitivity of a CT scan performed on the seventh day post-bleeding is only 50%.
- Hematocrit. The blood density appearing on CT depends directly on its hemoglobin concentration. Blood hemoglobin <10 mg/dl may be isodense on CT.
- Scanner resolution. The sensitivity of third-generation CT scanners to diagnose SAH is around 92-98%. It has been suggested that fifth-generation equipment has superior sensitivity which would approach 100%.
In some uncommon cases, blood doesn’t extravasate into the subarachnoid space but into the brain parenchyma, ventricular system or subdural space instead, causing intracerebral, intraventricular or subdural hemorrhages, respectively. A CT scan can also show false positive results as in cases of diffuse cerebral edema where congestion of the cerebral vessels occurs and appears on the scan as hyperdensity of the basal cisterns. This radiographic finding, called pseudo-subarachnoid hemorrhage, must be considered in the appropriate clinical context.
Magnetic Resonance Imaging
Brain magnetic resonance imaging (MRI) is an important ancillary study in the evaluation of patients with SAH. In MRI, unruptured aneurysms are hypointense on T1 and T2-weighted images and FLAIR (fluid attenuated inversion recovery) (Figure 51.2).
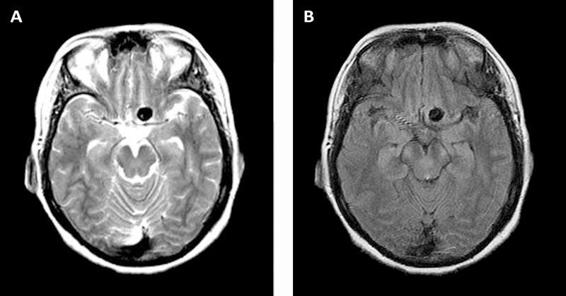
Figure 51.2. MRI obtained in a patient with an unruptured left internal carotid artery aneurysm. Axial T2-weighted-image (A) and FLAIR image (B).
It has been suggested that in the first 5 days, the sequences of proton density and FLAIR are as sensitive as CT for diagnosing the presence of blood in the subarachnoid space. In addition, FLAIR and GRE (gradient echo) sequences can demonstrate the presence of blood after day 4 with a sensitivity of 100% and 85%, respectively (Figure 51.3), a period in which the sensitivity of CT decreases significantly. The undisputed superiority of MRI over CT is that the former can locate not only the aneurysm responsible for SAH but also rule out other etiologies that make up the differential diagnosis, such as tumours or cerebral venous thrombosis which often go completely unnoticed on a CT scan.
MRI requires patients to remain motionless for a considerable amount of time. This limits its use in patients with an altered level of consciousness. In addition, MRI is contraindicated in patients with pacemakers or other implanted metallic devices which are incompatible with magnetic radiation.
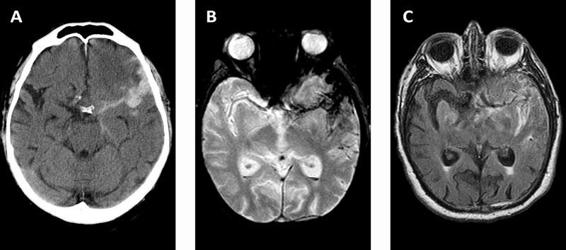
Figure 51.3. CT images (A) and MRI – GRE sequence (B) and FLAIR sequence (C) – obtained in a patient on day 4 after the rupture of a left middle cerebral artery aneurysm.
Ancillary Angiographic Studies
Ancillary angiographic studies such as magnetic resonance angiography (MRA) and three-dimensional computed angiotomography (CTA) are non-invasive diagnostic modalities that allow the identification of the vessel in which the aneurysm originates, the study of its anatomical configuration, and its anatomic relationship with other brain structures. These considerations are of vital importance when choosing the treatment modality (see below).
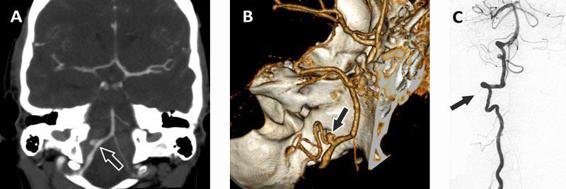
Figure 51.4. Images obtained in a patient with an aneurysm at the branching of the right postero-inferior cerebellar artery (PICA). A coronal reconstruction obtained by CTA (A), its digital 3D reconstruction (B), and the image obtained by DSA after the injection of contrast material in the right vertebral artery (C). The arrow indicates the location of the aneurysm.
Three-dimensional computed angiotomography. 3D CTA has considerably evolved recently. To visualize the intracranial vessels, a volume <100 ml of iodine contrast material is injected at a rate of about 4 ml/sec, and the area between the first cervical vertebra and the vertex is scanned. A 3D reconstruction of the intracranial vascular tree is created from the source images. The sensitivity of CTA varies with the size of the aneurysm. Compared to digital subtraction angiography (DSA), considered the gold standard for the detection of cerebral aneurysms, CTA has an average sensitivity of 95% (Figure 51.4). Correct evaluation with CTA will include not only a careful review of the vascular reconstruction but also the source images (Figure 51.5).

Figure 51.5. CTA images in a patient with an aneurysm of the left middle cerebral artery. Axial cut from the CTA source images (A), a coronal cut (B), and the digital 3D reconstruction (C). The arrow indicates the location of the aneurysm.
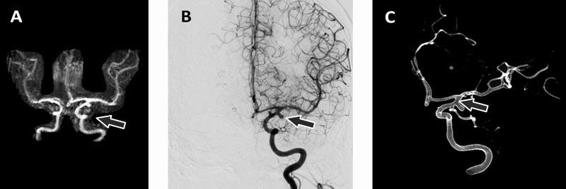
Figure 51.6. Images obtained in a patient with a left internal carotid artery aneurysm. MRA antero-posterior view after 3D reconstruction (A), DSA after the injection of contrast material in the left internal carotid artery (B), and DSA digital reconstruction (C). The arrow indicates the location of the aneurysm.
Magnetic resonance angiography. The three basic techniques used by MRA for the study of the cerebral vascular tree are the time of flight, phase contrast magnetic resonance angiography, and gadolinium infusion. They allow the visualization of the intracranial vasculature by acquiring two-dimensional or three-dimensional flow-sensitive imaging with background suppression. Three-dimensional images have better resolution and are therefore superior to two-dimensional images. Like CTA, MRA sensitivity depends on the size of the aneurysm. Compared to digital subtraction angiography, the average sensitivity of MRA is about 90-95% (Figure 51.6). MRA has the advantage of not exposing the patient to the potentially adverse effects of radiation and intravenous iodine contrast. However, the procedure requires the patient to remain motionless for a considerable amount of time, which limits its use in patients with psychomotor agitation. As in CTA, the interpretation of MRA should include a careful review of the vascular reconstruction and source images.
Digital subtraction intra-arterial angiography. DSA is considered the gold standard for detecting aneurysms. It should ideally be done within 24 hours of bleeding and should include the four large vessels (both internal carotid arteries and both vertebral arteries). As a general rule, when multiple aneurysms are observed, the largest, more irregular one is generally considered e the one responsible for the SAH as long as it matches the location suggested by the intracranial bleeding pattern. One of the main advantages of DSA is that, depending on the anatomical features, the same procedure can be used for endovascular treatment (see below). However, the technique is not devoid of potential complications. Its limitations include the use of radiation and contrast material, the risk of transient or permanent ischemic neurological complications, and re-rupture of the aneurysm, which are estimated at around 2% altogether.
Whatever the method chosen to investigate the source of SAH, it should carefully evaluate all cerebral vascular beds, given that up to 15% of patients will have multiple aneurysms. In those cases where angiographic studies fail to identify the source of the subarachnoid bleeding, we recommend repeating the study in 1 to 2 weeks. If in this instance an aneurysm is not found, an MRI study of the brain and cervical spinal cord should be obtained to investigate the possibility of vascular malformations of the brain, brainstem or spinal cord which may be partially thrombosed and hidden in the angiographic studies. The suggested diagnostic algorithm is shown in Figure 51.7.
Finally, it is important to note that a large percentage of the population over age 50 has small aneurysms (<5 mm) that are found incidentally and do not necessarily require treatment. Therefore, additional angiographic studies must be interpreted within the appropriate clinical context and reserved for the evaluation of patients in which SAH has been diagnosed.
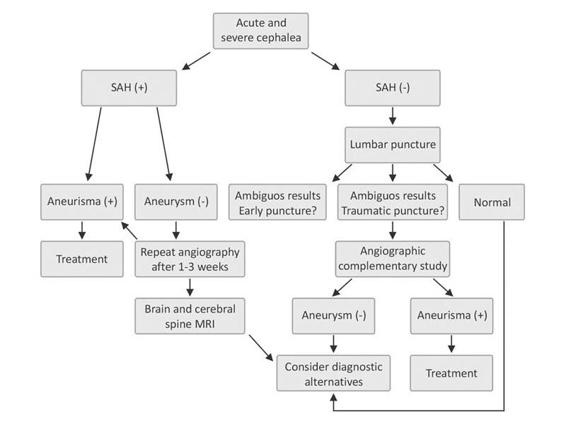
Figure 51.7. Suggested algorithm for the diagnosis of SAH.
51.3 Treatment and Management
In the approach to management of aneurysmal subarachnoid hemorrhage (SAH), consideration should be given to the clinical grade and initial presentation in each case. Resuscitative measures, airway, breathing and circulation, should be followed emergently with greater attention to unstable patients with worse neurological grades. Typically, all patients with aneurysmal SAH, irrespective of its grade, are admitted to our specialized ICU (Neurocritical Care Unit) where a combination of frequent neurological evaluation in addition to ICU monitoring protocols are instituted. It should be emphasized that patients with a low-grade SAH on presentation may rapidly deteriorate, making frequent neurological evaluation of paramount importance. Once the clinical suspicion of SAH is confirmed by CT or lumbar puncture, initial medical management should follow while diagnostic measures are being carried out. This helps to identify and treat potential medical factors that may contribute to neurological deterioration.
Airway. Low-grade SAH may manifest from a mild depressed mental state to a comatose state and in this setting there is a high risk of respiratory compromise due to poor or absent protective airway reflexes. Other factors that may compromise respiratory function include neurogenic pulmonary edema (discussed below) and trauma after falls due to sudden loss of consciousness or seizures. There should be a low threshold to intubation and initiation of mechanical ventilation in this setting. Attention should be paid to hypercapnia as this may worsen a frequent finding of raised intracranial pressure in this group of patients. Following intubation, anesthetic agents that decrease cerebral metabolism and also assist in blood pressure control such as propofol are reasonable. Other options include midazolam and fentanyl.
Blood pressure control. Most patients with aneurysmal SAH will present with elevated blood pressure due to pain, anxiety and sympathetic activation. A systolic pressure >160 has been associated with rebleeding or re-rupture, with an estimated mortality >50% in this sub-population. Several antihypertensives are available in the ICU setting, however, it is important to recognize the effects of each agent on cerebral autoregulation, predictable dose-response pattern, and safety profile. Continuous infusion of an antihypertensive such as nicardipine, a calcium channel antagonist, is our drug of choice in the initial acute setting, which is titrated to a set blood pressure range. The low end goal is to maintain adequate cerebral perfusion without increasing (high end goal) the risk of re-rupture or worsening intracranial pressure (ICP). Other options include labetalol and esmolol infusions. In cases where the blood pressure are relatively low (140-160 mmHg), intermittent intravenous labetalol and occasionally hydralazine (if there are no concerns of marked ICP elevations) are reasonable options. The use of nitroprusside is usually avoided in neurological emergencies due to its potential of worsening raised ICP and its toxicity with prolonged infusion, which is sometimes necessary in these clinical settings.
Bed rest. Historically, bed rest in a quiet surrounding has been prescribed in patients with aneurysmal SAH under the assumption that any form of excitement would increase the risk of rebleeding due to surges in blood pressure. In addition to the management of pain and anxiety, it constitutes part of our treatment protocol.
Pain and anxiety. Acute SAH presents as a sudden severe headache and has been described as “the worst headache in my life”. Therefore, it seems reasonable that the accompanied surge in catecholamines results in an increase in sympathetic activity with an antecedent rise in blood pressure. In non-intubated patients we have found low-dose opioids (e.g., morphine) to be helpful in this regard. We specifically avoid non-steroidal anti-inflammatory drugs (NSAIDs) due to the increased risk of bleeding from their anti-platelet effect.
Antifibrinolytic therapy. Studies of antifibrinolytic agents, such as epsilon aminocaproic and tranexamic acid, in aneurysmal SAH demonstrated a reduction in rebleeding; however, an increased incidence of thromboembolic events offset any benefit. In the majority of studies, antifibrinolytic agents were used for at least 96 hours. In a Swedish study comparing the use of tranexamic acid initiated within a mean of 4.9 hours from symptom onset, until the aneurysm was secured, and given for <72 hours, the incidence of rebleeding was lower in the treatment group. There was no difference in thromboembolic events or delayed cerebral ischemia in this study. We have occasionally used short-term antifibrinolytic agents in highly selected patients for a short duration if a delay in securing the aneurysm is anticipated as may occur in medically unstable patients or in the long-distance transfer of SAH patients. These agents are then held 6-8 hours prior to securing the ruptured aneurysm. However, our preference is to achieve aneurysm occlusion within 24 hours of symptom onset, obviating the need for antifibrinolytic agents in most cases. It seems that this approach accomplishes the goal of preventing rebleeding and obviates the need for antifibrinolytic agents and their potential complications.
Seizure treatment and prophylaxis. The frequency of seizures after SAH is reported to be 6-20%, with about half of episodes occurring during the perioperative period. Risk factors associated with seizures in retrospective series include middle cerebral artery aneurysm, intraparenchymal or subdural hematoma, cerebral infarcts, thickness of the SAH clot, and hypertension. Often, seizures manifest with rhythmic motor movements and are associated with altered state of consciousness. Persistent loss of consciousness is worrying and should be aggressively evaluated for non-convulsive status epilepticus or raised ICP. We have frequently encountered patients with persistent alterations of consciousness who were reported to have had recurring “seizure-like” events after SAH that were non-epileptic on the electroencephalogram (EEG) and persisted despite adequate anticonvulsant medication. It appears that some of these events may be manifestations of cerebral posturing due to a sudden sustained rise in ICP, known as plateau waves. However, since seizures have been associated with intracranial injury, it is important that they be aggressively treated especially in cases of status epilepticus. The initial management involves the use of benzodiazepine (e.g., lorazepam and diazepam) and an anticonvulsant such as phenytoin or valproic acid. The use of new intravenous agents such as levetiracetam and lacosamide remain adjunct options. When there is no improvement in the level of consciousness or seizures persist, continuous EEG monitoring is a helpful tool for differentiating epileptiform from non-epileptiform events but it should not deter continuing management of status epilepticus. The presence of status epilepticus typically warrants long-term anticonvulsant treatment with outpatient follow up.
In seizure prophylaxis, there have been very few reports suggesting its benefit especially in the long term. There is some concern about worse cognitive outcome at 3 months with phenytoin reported in a few series. The major argument for seizure prophylaxis is that it decreases the risk of rebleeding from an unsecured aneurysm due to a seizure event. This theoretical benefit should be weighed along with the potential long-term effects by selecting patients with the above mentioned risk factors for seizures. When seizure prophylaxis has been instituted, limiting it to a duration of 7-14 day appears reasonable. We have occasionally continued prophylaxis for a longer period in patients with epileptiform discharges on EEG but without seizures and temporal hematoma associated with an middle cerebral artery aneurysm.
Nimodipine. Nimodipine has been demonstrated to improve functional outcome after aneurysmal SAH. It is typically given at doses of 60 mg every 4 h. It can cause hypotension and headache; the hypotensive effects are beneficial in the initial stages when blood pressure control is important. Subsequently, as one nears the vasospasm period when hypotension needs to be avoided, its use becomes challenging. Its benefit appears to be related to cerebral neuroprotection since its effect on vasospasm is minimal. This is further discussed in the section “Vasospasm” below.
Fever. See below.
51.3.1 Surgical and Endovascular Management
Patients with aneurysmal SAH are at an increased risk of re-rupture of the patent aneurysm (3-4% in first 24 h and 1-2% daily for 30 days) with a mortality rate >55%. The definitive approach to preventing further clinical deterioration from re-rupture involves securing the aneurysm via endovascular coiling or surgical clipping. Several studies have suggested that an increased time to securing a ruptured aneurysm increases the risk of rebleeding. However, in the International Cooperative Study on the Timing of Aneurysm Surgery, the overall outcome was not significant. Our approach is to secure the aneurysm as soon as possible with either surgical or endovascular techniques. In our opinion, this allows for easier management of vasospasm, a known and common complication of aneurysmal SAH.
The advent of endovascular treatment of intracerebral aneurysms with detachable coils has complemented and in some situations replaced surgical clipping in securing a ruptured aneurysm. The decision on which method of securing the aneurysm depends on several factors such as patient age, clinical grade, location (anterior vs. posterior circulation), aneurysm size and morphology, presence of other vascular injuries (e.g., intracerebral or subdural hematoma), procedural efficacy and facility-specific characteristics (availability of technical and infrastructure support).
Endovascular Management
This treatment technique involves deploying platinum coils through microcatheters into an aneurysm with the goal of achieving complete obliteration and prevent future rebleeding. This relatively minor invasive approach to treatment has made coiling a more attractive technique in securing aneurysms. The effectiveness of the procedure in the treatment of ruptured aneurysm can be measured by the procedural efficacy (rebleed rate and angiographic recurrence rate of a treated aneurysm). Comparison with surgical clipping in the International Subarachnoid Aneurysm Trial (ISAT) found a relative risk reduction of death or dependency by 23.9% at 1 year in the endovascular group. However, the rebleed rate at 1 year in the endovascular group was 2.9% vs. 0.9% in the surgical clipping group. The long-term outcome in this study, (mean follow-up, 9 years), suggested a non-significant increase in rebleed rate in the endovascular group. Murayama et al., in a series with a follow-up of over 11 years, reported that 55% of aneurysms treated with endovascular coiling could be completely occluded. The factors affecting incomplete occlusion and recurrence were aneurysm size and shape. It is important to note that aneurysm size and shape were also identified as predictors of rebleeding.
An anticipatory approach to the care of patients prior to an endovascular procedure is critical. Attention should be paid to adequate airway and ventilation management, blood pressure control, avoiding anesthetic agents (e.g., ketamine) and hypercapnia that may increase intracranial pressure. If an ICP monitor is already in place, close monitoring of ICP changes and especially the ICP waveform as a predictor of intracranial compliance and peri-procedural complication is informative. In the absence of an ICP monitor, sudden blood pressure spikes or evidence of procedural aneurysmal rupture may present an emergent need for placement of an ICP monitor with external CSF diversion. Other medical measures in treating raised ICP with hyperosmolar agents (mannitol, hypertonic saline), sedative anesthetic (benzodiazepine, barbiturates) may be considered in refractory elevated ICP. In some cases, the need for stent placement in an attempt at vascular reconstruction while securing an aneurysm may arise. In this circumstance, the subsequent need for antiplatelet therapy makes this an important determining factor in the timing of invasive procedures such as placement of external ventricular drains (EVD). Also of interest is the development of intra-arterial thromboembolism during endovascular coiling. Several case series have reported treatment of this complication with a glycoprotein IIb-IIIa antagonist such as abciximab with relative good outcomes. The frequency of aneurysmal rebleed and primary intracerebral hemorrhage in these series has been low, though hemorrhagic conversion of a cerebral ischemic lesion has been described. These patients should be monitored closely for neurological deterioration.
The selection of patients for endovascular coiling is therefore important so as to optimize benefit and reduce the above mentioned risk following treatment. In the ISAT study, the mean age was 52 years with older patients (>70 years) preferably undergoing endovascular coiling due to the higher surgical risk. For the same reason, we typically will consider endovascular treatment for patients older than 50 years, especially in a setting of multiple medical conditions that preclude surgical clipping. Other features that are optimal for endovascular coiling include: aneurysms in the cavernous segment of the internal carotid artery aneurysm, internal carotid artery and posterior circulation (e.g., basilar tip), aneurysm neck diameter <5 mm, and a neck diameter to largest aneurysm dimension ratio <0.5.
In recent years, following the publication of the ISAT study, endovascular treatment of intracranial aneurysms has gained wider acceptance. Techniques and materials have improved significantly, so that wide-necked aneurysms which were once impossible to embolise can today be occluded with the help of balloons or stents (balloon-assisted coiling, stent-assisted coiling). However, the largest concern is the fact that on subsequent follow-up angiography some of the endovascular-treated aneurysms have grown due to initial incomplete filling of the aneurysm sac or have compacted coils in the dome of the aneurysm as a result of the water hammer effect generated by pulsatile blood flow allowing the neck to eventually grow and increase the risk of a re-rupture. This means that a significant proportion of these aneurysms require retreatment by adding more coils (with the risk associated with this new procedure). A slightly increased risk of re-rupture of coiled aneurysms when compared with surgically clipped ones has also been reported.
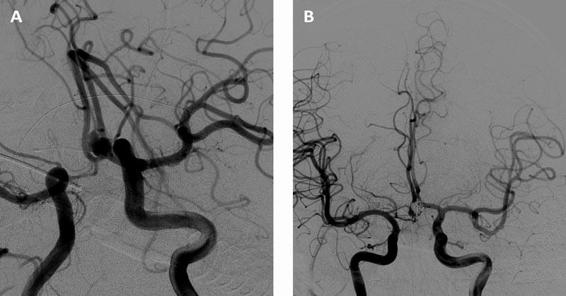
Figure 51.8. Cerebral DSA showing an aneurysm of the anterior communicating artery (A). Cerebral DSA immediately after endovascular occlusion of the aneurysm with platinum coils (B).
Surgical Management
Cerebrospinal fluid diversion and aneurysmal clipping are the main surgical treatments of intracranial aneurysms. The use of CSF diversion techniques is important at different stages in the treatment of aneurysmal SAH. At initial presentation, poor-grade SAH may be secondary to acute hydrocephalus requiring emergent ventriculostomy. Other CSF diversion techniques are typically reserved for late complications of SAH (discussed below). Though surgical clipping is a more invasive procedure, it accomplishes complete obliteration of the aneurysm in 91.8% of cases.
Ventriculostomy. This procedure involves the placement of a soft catheter into the ventricle to allow ICP measurement and CSF drainage. This can be done at the bedside or in the operating room during surgical clipping of the aneurysm. The benefit of an external ventricular drain stems from the experience that not all poor-grade SAH are immediately catastrophic due to complete cerebral circulatory arrest. Some 20-30% of patients with aneurysmal SAH have acute hydrocephalus and may manifest as poor-grade SAH. We emergently place an EVD in all our poor-grade patients to measure and relieve the intracranial pressure via cautious CSF drainage. This improves the clinical grade of some patients and thus decreases mortality. Acute deterioration from a good grade SAH may also result from acute hydrocephalus and is clinically suggested by worsening mental status, upgaze palsy, slow pupillary response, bilateral 6th cranial nerve palsy and bilateral lower extremity spasticity. Hence, the indications for an EVD in aneurysmal SAH are hydrocephalus, intraventricular hemorrhage, and poor-grade SAH. A theoretical advantage has been suggested in placing an EVD in patients with large amounts of SAH. Clearing of the bloody CSF may decrease the severity of vasospasm, but this remains to be proven clinically. When we use this approach, it is not done at the expense of increasing the risk of infection due to prolonged EVD use. Opponents to this theory suggest that the CSF drained is primarily from the ventricles where it is produced, thereby discouraging drainage of subarachnoid blood in the cisterns via their natural pathways.
As with every procedure in the intensive care unit, EVD insertion carries risks – aneurysmal rebleeding, infection (ventriculitis), and intracerebral hemorrhage. Theoretically, the concern about aneurysmal rebleeding is due to relieving the tamponade effect of elevated ICP on the transmural wall pressure of the aneurysm. Our practice has been to set a high pressure CSF drainage level (e.g., 20-25 mmHg) to avoid this complication in patients with unsecured aneurysms while preserving cerebral perfusion pressure. Placing an EVD under strict sterile technique, maintaining a closed system, and timely decision on removing the EVD are important factors that help in minimizing infection. Other preventive measures include the use of antibiotic-coated catheters or antibiotic prophylaxis (e.g., cefazolin 1 g/8 h or clindamycin in patients with penicillin allergy) which are continued for the duration of the EVD. This, however, is a controversial issue: it decreases the rate of ventriculostomy-associated infections but increases the incidence of methicillin-resistant Staphylococcus aureus (MRSA) and Gram-negative infections. However, we have found daily CSF surveillance for glucose, protein, cell count, gram stain and culture to be helpful. A low glucose level with or without CSF positive cultures is highly suspicious of CSF bacterial infection. Prompt removal of the EVD and broad-spectrum antibiotics are our initial aggressive approach. Intracerebral hemorrhage complicating EVD insertion is commonly seen in the setting of an underlying coagulopathy, rupture of a bridging vein or cortical vessel and multiple passes or attempts to drain the ventricles. In non-emergent circumstances, we aim to correct the underlying coagulopathy or thrombocytopenia (INR <1.4 and platelet >100,000/μl) prior to inserting an EVD. In the case of an emergency EVD, we have found transfusing with fresh frozen plasma and/or platelets, as required before, during and after the procedure, to be helpful in reducing these complications. Factor VII could also be an option in these settings, but the cost limits its availability in some centres.
Surgical clipping. This surgical procedure accomplishes a higher rate of aneurysm obliteration as compared to endovascular repair and hence less need for re-exploration of the aneurysm. In the ISAT study, surgical clipping demonstrated a lower rebleed rate compared to aneurysm coiling. Also, the study showed that the need for retreatment of the aneurysm persisted in the long term after coiling as opposed to surgical clipping. It appears that surgical clipping was more beneficial in younger patients and larger lumen aneurysms. Among these features, other patient or aneurysm characteristics that favour surgical clipping include middle cerebral artery aneurysm, very small or large to giant aneurysms, wide neck aneurysm, associated intracerebral hemorrhage or parenchymal hematoma, and failed endovascular coiling. Despite the apparent advantage in securing a ruptured aneurysm, not all patients are eligible for surgical treatment. Relative exclusion criteria include poor clinical grade or complicated medical history, severe diffuse brain swelling that limits retraction during surgery, posterior circulation aneurysms due to their technically difficult access.
Another advantage of surgical clipping is the ability to create an internal CSF diversion by fenestration of the lamina terminalis during the surgical procedure. Some case series have suggested a decrease in the incidence of chronic hydrocephalus requiring a CSF shunt after this procedure. Uncommonly, decompressive craniectomy may be offered in the setting of severe diffuse cerebra edema, especially when there is associated intracerebral hemorrhage.
Post-operative care is geared towards:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






