Chapter 17 Nondegenerative Diseases of the Spine
INFECTIONS AND INFLAMMATORY DISEASES
Diskitis and Osteomyelitis
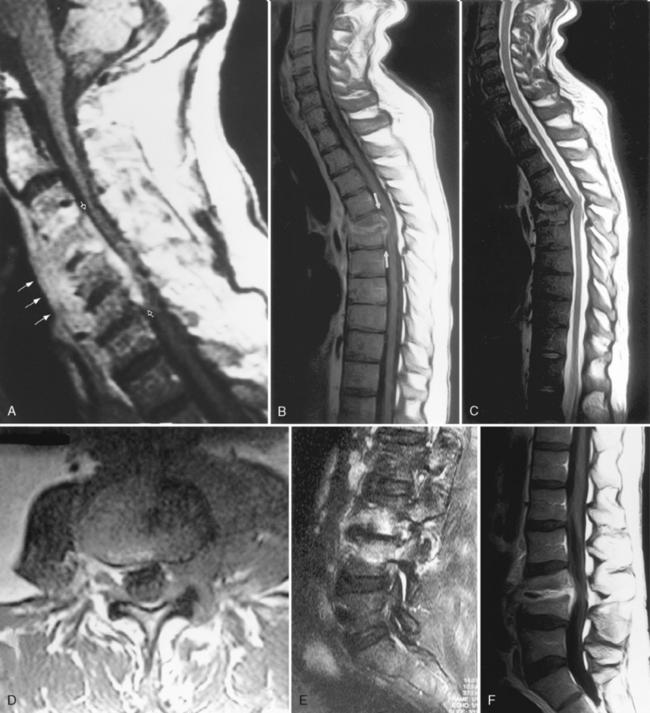
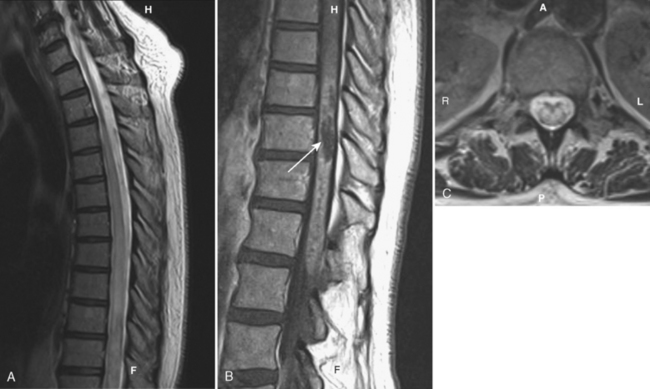
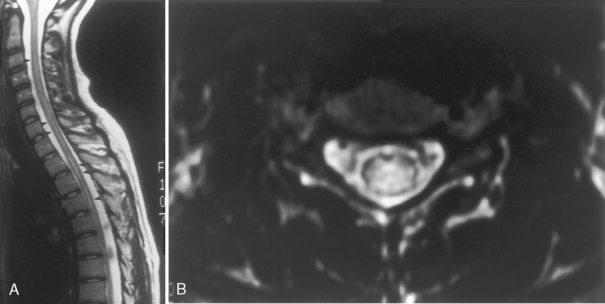
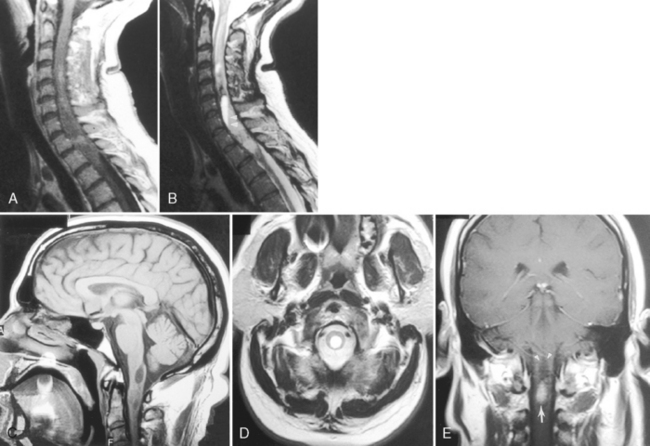
Although gallium- and indium-labeled white blood cell radionuclide imaging is highly sensitive to inflammation, magnetic resonance (MR) imaging is the most specific method for cost-effective diagnosis. Conventional spin-echo images reveal the disk space to be of low signal intensity on T1-weighted images (T1WI) and high signal intensity on T2-weighted images (T2WI) as a result of the associated edema (Fig. 17-1). The disk space, adjacent vertebral bodies (particularly at the endplates), and, if present, the epidural or paravertebral soft tissues enhance. Unfortunately Modic type 1 marrow changes simulate those seen in diskitis/osteomyelitis and may enhance. High T2WI signal in the endplates of the vertebral bodies is the least reliable of the findings for diskitis/osteomyelitis. Erosions of the endplates and gadolinium enhancement of the disks become paramount. There is usually evidence of irregularity of the endplate or bony sclerosis (best seen on computed tomography [CT]). Look carefully for epidural or paravertebral abscesses.
Granulomatous Infections
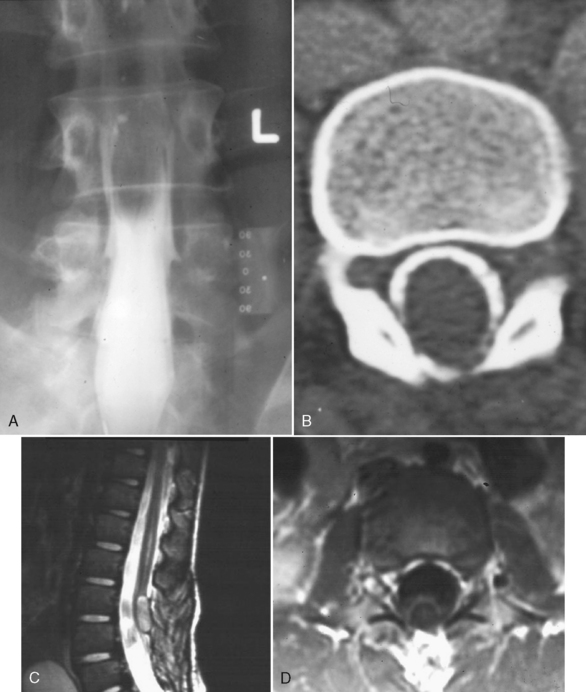
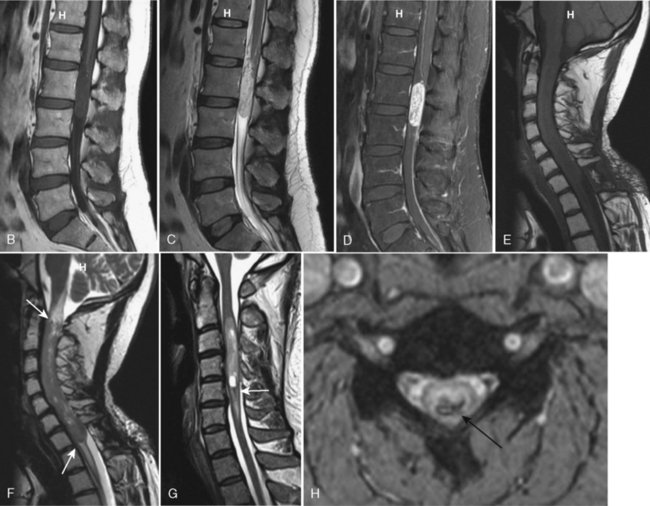
Tuberculosis (TB) most often affects the lower thoracic spine (Pott disease, tuberculous spondylitis), is indolent, and is frequently associated with epidural disease, particularly paravertebral, subligamentous, or psoas muscle abscesses. Vertebral body destruction with relative sparing of the disk space occurs late in the disease (Fig. 17-2). Because of the infection, a gibbus deformity may develop. The posterior elements may be involved, and the infection can spread along the anterior longitudinal ligament and involve multiple levels, spreading anteriorly. The disk can be of normal intensity on noncontrast MR but usually enhances. Spinal deformity is common. Approximately 50% of patients have obvious pulmonary disease. The diagnosis of TB requires isolation of the bacillus, characteristic histopathologic changes, or response to antibiotic therapy. Table 17-1 contrasts the features of bacterial infection, TB, and neoplasm. The usual differential diagnosis includes sarcoidosis, fungus, and brucellosis, but a calcified inflammatory mass, particularly one that affects the psoas muscle in the lumbar area, is classic for TB.
Epidural Abscess
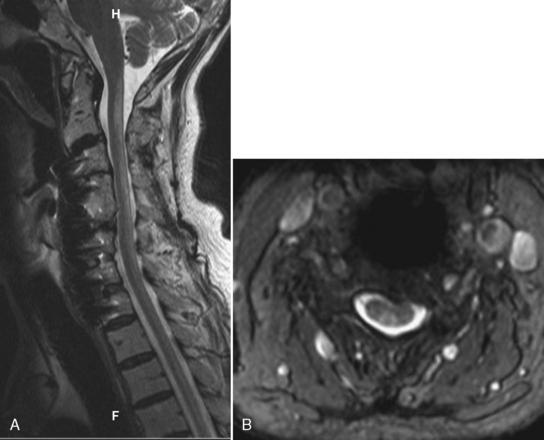
MR is the modality of choice for imaging epidural abscesses (Fig. 17-3). The epidural mass has low signal intensity on T1WI and has high signal on T2WI. Uncomplicated, the cord has normal signal. There are three patterns of enhancement: (1) homogeneous enhancement, representing diffuse inflamed tissue with microabscesses (phlegmonous granulation tissue); (2) peripheral enhancement consistent with frank abscess, including a necrotic center; and (3) a combination of tissue enhancement and frank abscess. The first pattern may be difficult to discern in the lumbar spine without the use of fat-saturation techniques to distinguish between enhancement and epidural fat, which can be diffusely infiltrated by the inflammatory process. Epidural abscess produces symptoms by sheer mass effect or septic thrombophlebitis with cord edema and infarction. Think about spinal cord infarction secondary to thrombophlebitis in the setting of epidural abscess and high signal on T2WI in the cord. Meningitis (with increased intensity of the cerebrospinal fluid [CSF] on T1WI and enhancement of the CSF and nerve roots) can occasionally be identified along with the epidural abscess.
Meningeal Sequelae
Infections of the meninges are discussed in Chapter 6. The sequela of bacterial meningitis may be arachnoiditis, which at times is associated with syringomyelia or acquired arachnoid cysts (see section on Cystic Lesions in this chapter). In addition, cysticercosis can cause arachnoiditis (and rarely subarachnoid and spinal cord cysts). Pachymeningitis (thickened dura mater) has many causes (Box 17-1).
Sarcoidosis
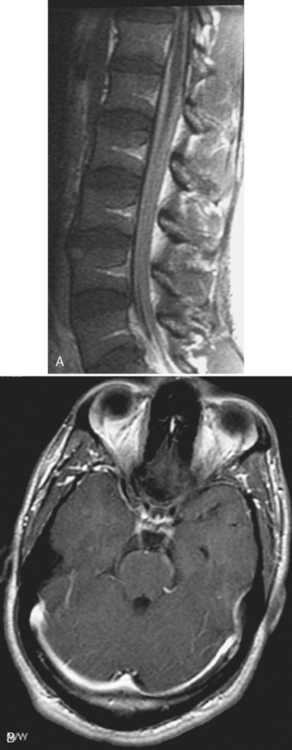
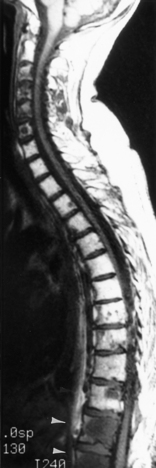
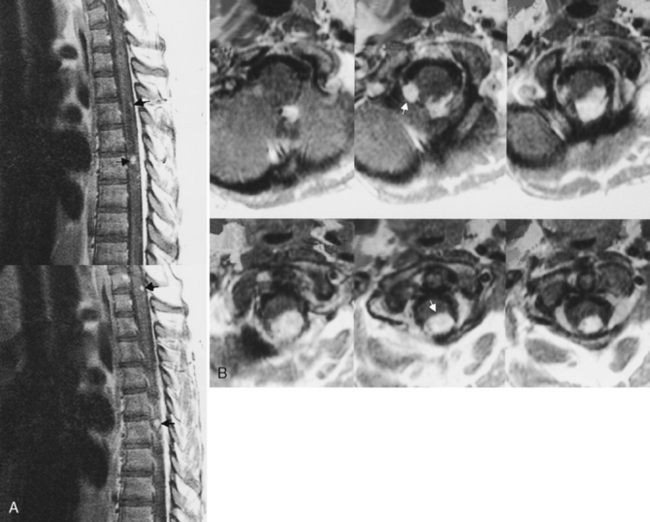
Only 6% to 8% of patients with neurosarcoidosis have spinal cord lesions, with the most common location being the cervical region. Sarcoidosis can manifest as nodules on the surface of the cord, which can appear similar to tumor nodules from subarachnoid seeding and can be associated with cord enlargement. Rarely, sarcoid nodules may be on individual nerve roots. As in the brain, intramedullary lesions result from infiltration of the Virchow-Robin spaces (Fig. 17-4). Intramedullary sarcoidosis can present as a diffuse inflammatory lesion or as a discrete mass. Unfortunately, patients with sarcoidosis on or off steroids have an increased rate of spinal TB or fungal infection, which obscures matters further.
Intramedullary Lesions in Acquired Immunodeficiency Syndrome
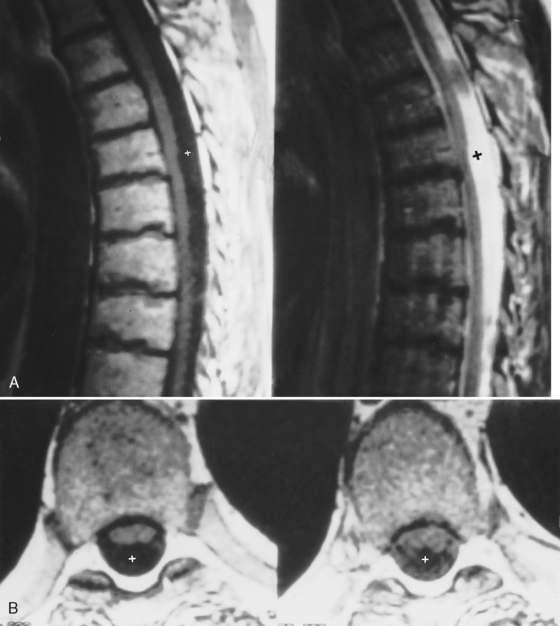
A variety of conditions affect the cord in patients with acquired immune deficiency syndrome (AIDS); these are listed in Box 17-2. Vacuolar myelopathy, identified in patients with AIDS (AIDS-associated myelopathy), is defined as vacuolation in the spinal white matter associated with lipid-laden macrophages, typically involving the dorsal columns and lateral corticospinal tracts, although it may occur anywhere (Fig. 17-5). Degeneration of the corticospinal tract has also been reported, especially in children. Its incidence has been reported to be between 3% and 55%. The etiology is unknown but may be related to either primary or indirect effects of human immunodeficiency virus (HIV), secondary infectious agents, nutritional deficiencies (especially of vitamin B12), or toxic effects of drugs. The vertebral marrow can also be affected by these conditions. High intensity on T2WI is seen in a spinal cord that may be large, normal, or small. Nothing is specific about the MR appearance to separate AIDS-associated from other non-AIDS intramedullary lesions.
Transverse Myelitis and Multiple Sclerosis
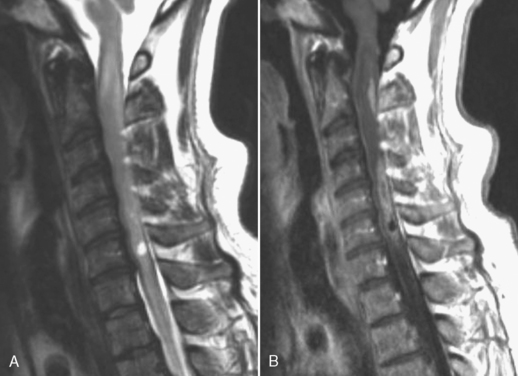
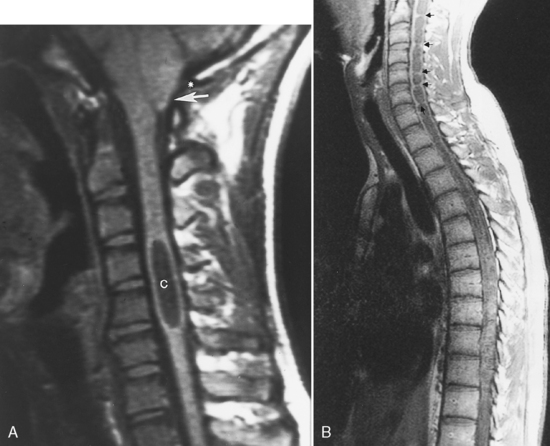
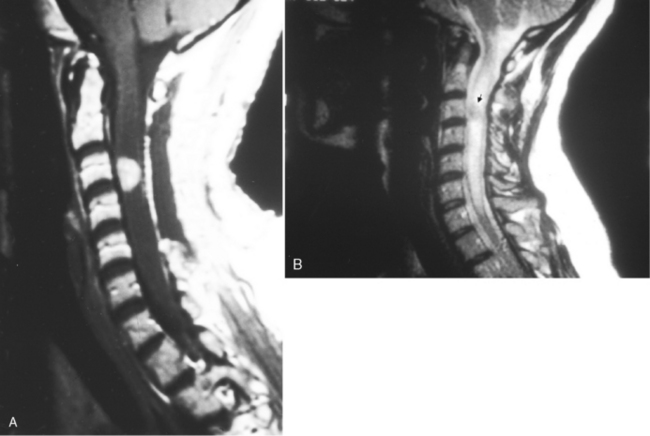
Transverse myelitis is an inflammatory condition of the spinal cord associated with rapidly progressive neurologic dysfunction, usually with numbness, paresthesias, and paraparesis. The term indicates either bilateral dysfunction of the spinal cord or partial unilateral abnormality. Diseases causing this condition include acute disseminated encephalomyelitis (see Chapter 7), multiple sclerosis, connective tissue diseases (lupus, rheumatoid arthritis, and Sjögren disease), sarcoidosis, vascular malformations, and vasculitides. Idiopathic cases also exist. The spinal cord is focally enlarged with high signal on T2WI and may enhance (Fig. 17-6).
With idiopathic acute transverse myelitis, the clinical course occurs over days to weeks. Pathology reveals demyelination, perivascular lymphocytic infiltrates, and necrosis. The lesion extends over multiple spinal cord segments and involves the entire cross-section of the spinal cord. There is high intensity on T2WI with variable enhancement patterns (nodular, meningeal). In some cases, the cauda equina enhances, suggesting a possible relationship with Guillain-Barré syndrome. It has been hypothesized that this is a result of a small-vessel vasculopathy (perhaps immunologically mediated), either arterial or venous, affecting gray matter as well as white matter (Fig. 17-7).
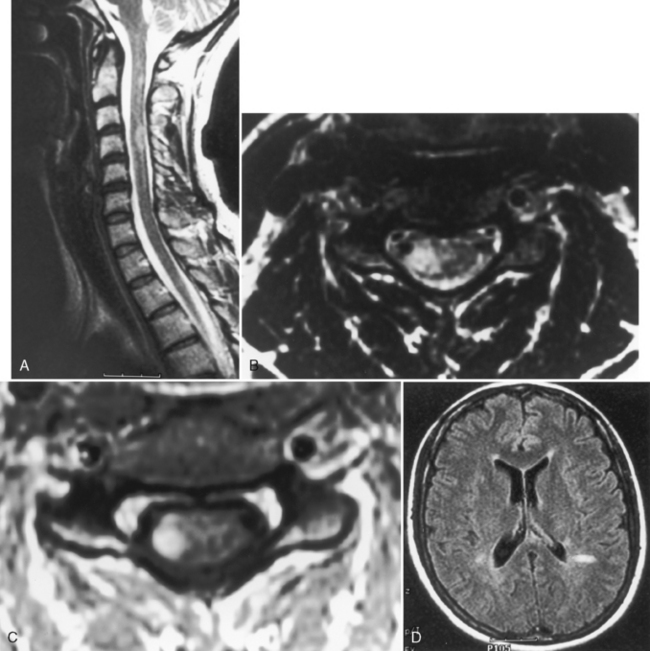
Multiple sclerosis (MS) affecting the spinal cord is visible on MR in 70% to 80% of patients and produces myelopathic signs and symptoms. It can be confined solely to the spinal cord (5% to 24% of cases), which may account for the “negative” brain MR findings in patients with definite disease. MS lesions are isointense or of low intensity on T1WI and high signal on T2WI. Sixty percent of the spinal cord lesions occur in the cervical region. The typical MS spinal cord lesion does not involve the entire cross-sectional area, is peripherally and posteriorly located, does not respect gray-white boundaries, and is less than two vertebral body segments in length (90% of the time). The cord is usually normal in size, with enlargement seen in 6% to 14% of cases (Fig. 17-8). The majority of lesions are patchy in location and demonstrate enhancement if the patient is referred for problems related to new spinal cord signs/symptoms. Spinal cord parenchymal loss, especially in the cervical region, can be detected over the course of the disease. Lesions in the spinal cord account for less than 20% of total disease burden. Nevertheless, a strategically placed lesion can cause profound disability. One other point: MS is a common disease, so beware of unusual presentations, particularly in young patients.
It is useful to image the brain if questions are raised concerning the cause of the transverse myelitis, because asymptomatic high signal abnormalities in young adult brains help favor MS as the cause of the transverse myelitis. Try the sagittal fluid-attenuated inversion recovery (FLAIR) scan and look at the callosal-septal interface (Fig. 17-8D).
Lupus Myelitis
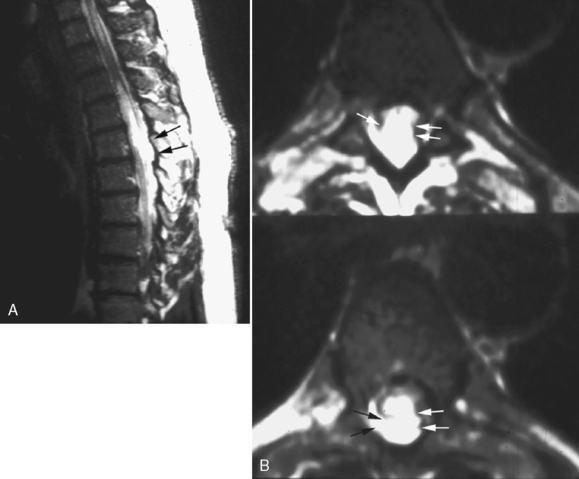
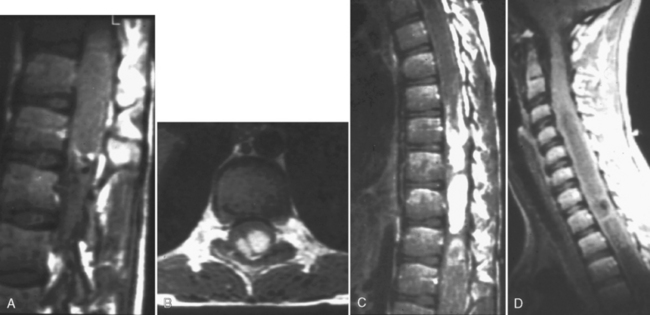
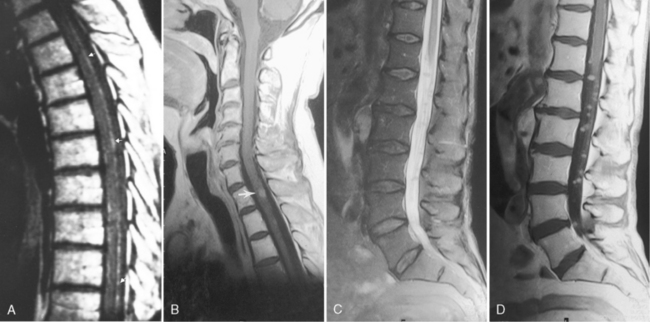
This is a recognized complication of systemic lupus erythematosus presenting as transverse myelitis with back pain, paraparesis or quadriparesis, and sensory loss. The spinal cord may be enlarged with high intensity on T2WI involving four to five vertebral body segments (more than in MS) of the cord (Fig. 17-9). Contrast enhancement can be detected in approximately 50% of cases. Etiology of the lesion is vacuolar degeneration from an autoimmune process or ischemia.
Subacute Combined Degeneration
Box 17-3 provides the differential diagnosis of degenerative lesions involving the posterior columns. This includes nitrous oxide toxicity, copper deficiency, and vacuolar myelopathy. On T2WI, high intensity is observed longitudinally in the posterior columns (Fig. 17-10). After treatment, this can regress and disappear if caught early enough; however, if undetected, permanent axonal loss and gliosis results. The bone marrow can also be low intensity on T1WI and T2WI associated with enhancement, indicating benign hyperplasia of the hematopoietic marrow (secondary to the pernicious anemia).
RADIATION-INDUCED CHANGES
Spinal radiation converts normal bone marrow to fatty marrow (Fig. 17-11). This is manifested on MR by diffuse high signal on T1WI in the vertebral bodies and lower intensity on conventional T2WI. If the cord is high signal on T2WI, it may be the result of radiation myelitis or residual tumor, if the reason for the initial therapy was a cord tumor. Cord atrophy is another manifestation of radiation therapy. Box 17-4 provides a list of conditions that produce increased or decreased marrow signal on T1WI.
CYSTIC LESIONS
Arachnoid Cyst
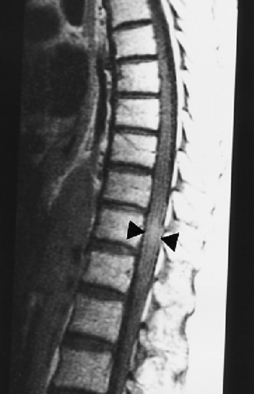
Arachnoid cysts occur on a congenital basis or result from trauma, postoperative scarring, or inflammation. On CT myelography, you can see an extramedullary mass, which may compress the cord. The cyst may or may not fill with contrast on delayed imaging. On MR focal impression on the cord can be seen with intensity similar to that of CSF without enhancement (Fig. 17-12). Carefully scrutinize the smoothness of the cord; any indentation suggests the presence of arachnoid cyst. These cysts can be under pressure and produce a myelopathy.
Tarlov Cyst
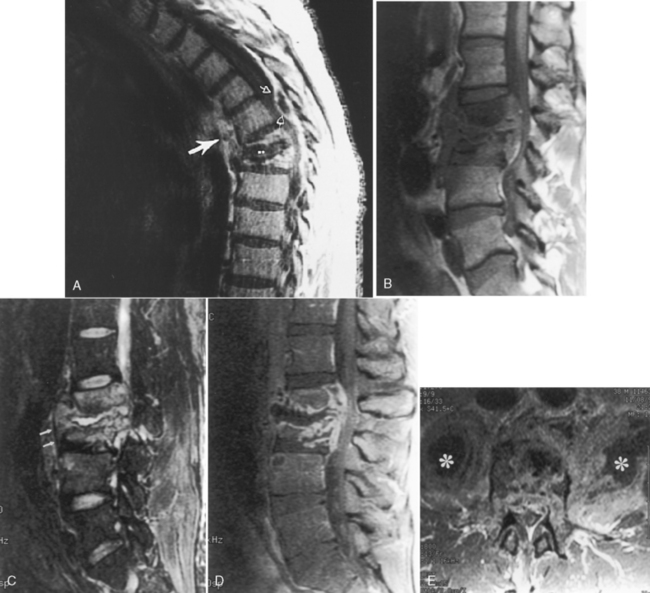
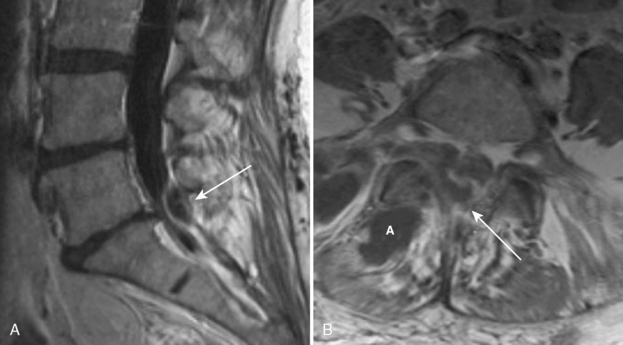
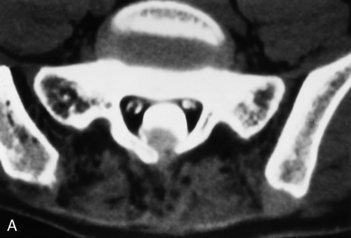
Cystic dilation of the sacral root pouches (Tarlov cysts) can be large and may be associated with bone erosion (Fig 17-13). They occur in about 5% of individuals and vary in size from a few millimeters to several centimeters. These are caused by a ball-valve phenomenon at the ostium of the nerve root sheath with CSF flowing into the cyst with arterial pulsation. After instillation of subarachnoid contrast, these cysts may or may not fill. These may or may not be symptomatic (rare cause of sciatica). Those that are symptomatic and do not readily communicate with the subarachnoid space may be treated with fibrin glue ablation. The differential diagnosis includes meningocele, arachnoid cyst, neurofibroma, and dural ectasia. The Nabors classification of spinal meningeal cysts includes extradural cysts without spinal nerve roots (type I), with spinal nerve roots (type II [Tarlov cysts]), and intradural cysts (type III, intradural arachnoid cysts). Anterior sacral meningoceles herniate into the presacral space and are usually unassociated with nerve roots and as such are type I cysts. When there is an associated anorectal malformation (imperforate anus), bony sacral defect (scimitar sacrum), and presacral mass (cyst/teratoma/meningocele), the term Currarino’s triad is used.
Epidermoid Cyst
Epidermoid cysts represent less than 1% of intraspinal tumors, with a higher incidence in children. They are usually extramedullary but rarely can be intramedullary, and can be congenital or acquired. Congenital epidermoids result from displaced ectoderm inclusions occurring early in fetal life, perhaps from faulty closure of the neural tube, usually in the lumbosacral region. The acquired cysts result from lumbar puncture with inclusion of skin tissue in the spine. On MR, a discrete mass, which has variable signal intensity depending on the cyst contents, is present (Fig. 17-14). The lesion can calcify and can be associated with peripheral enhancement in rare instances. Inclusion of endoderm results in an enterogenous cyst that is lined by mucin-secreting cells, which can produce contents with similar intensity (high signal on T1WI and T2WI) to mucoceles. These can be associated with developmental defects in the skin and vertebral bodies, and with fistulous communications to cysts in the mediastinum, thorax, or abdomen. Other cysts include dermoid cysts (which contain fatty elements) and ependymal cysts (which follow CSF intensity). A dermal sinus tract may coexist in up to one fourth of cases, in which case the epidermoid or dermoid may be both intramedullary and extramedullary.
Syringomyelia (Syringohydromyelia)
There are three types of syringohydromyelia. The first is a central canal syrinx that communicates with the fourth ventricle and is associated with hydrocephalus. These are produced by obstruction of CSF circulation distal to the outlets of the fourth ventricle. The second occurs in a region of the central canal that is dilated but does not communicate with the fourth ventricle. Here we are referring to those associated with Chiari I malformations, intradural extramedullary tumors, arachnoid cysts/arachnoiditis, cervical spinal stenosis, basilar invagination, and so on (Fig. 17-15; Box 17-5). The first and second cases have also been termed hydromyelia. There may also be cases of asymptomatic localized dilation of the central canal without any predisposing factors such as Chiari malformation (there are exceptions to any theory).
The third differs from the first two in that it is centered in the spinal cord parenchyma and is not centered in the central canal (Box 17-6). These are found in watershed regions of the spinal cord and associated with direct spinal cord injury such as trauma or infarction. This is usually termed a syrinx.
Rarely an eccentric intra- and extramedullary syrinx can be identified. This can be confused with arachnoid cyst. One wonders if at least a few of the arachnoid cysts noted with syringomyelia may actually be eccentric syrinxes. An evaginated syrinx has been termed an exosyrinx (Fig. 17-16).
Cysts Associated with Neoplasia
Neoplasms of the spinal cord commonly have cysts associated with them. These cysts have been termed tumoral (or intratumoral) and nontumoral (reactive) (Fig. 17-17). Cysts rostral or caudal to the solid portion of the tumor (nontumoral) are secondary to dilation of the central canal. They do not enhance, are not echogenic on intraoperative ultrasound, do not have septations, and usually disappear after resection of the solid lesion. Allegedly, fluid produced by the tumors dilates the central canal, producing the cyst. Tumoral cysts (more common in astrocytoma) occur within the tumor and may show peripheral enhancement.
NEOPLASTIC DISEASES
Intramedullary Lesions
Magnetic resonance imaging has had a huge impact on the diagnosis of neoplastic diseases of the cord. Spinal cord tumors represent approximately 5% of central nervous system (CNS) neoplasms. Neoplasms have three general characteristics: (1) They tend to enlarge the cord either focally or diffusely, (2) on T2WI they produce high signal intensity, and (3) they nearly always enhance (Fig. 17-18). Tumors involving the cord may be primary, such as astrocytoma or ependymoma, or metastatic. It is virtually impossible to separate ependymoma from astrocytoma, but we will provide tricks of the trade to improve the coin flip. Symptoms include pain, weakness, and muscle atrophy. Box 17-7 provides the differential diagnosis of the enlarged spinal cord. This is an essential diagnosis to know—memorize it! You must appreciate, however, that not all enlarged cords are neoplasms. As can be appreciated from Box 17-7, inflammatory and demyelinating diseases may enlarge the spinal cord. Especially in young patients with acute or subacute myelopathic symptoms, you should not glibly suggest that an enlarged spinal cord with high T2 signal represents a tumor. Rather, think about the case, assess presence of enhancement (absence unusual in tumors), get an MR of the brain, and assess whether there are additional lesions. In this patient population, corroborative findings might favor a demyelinating process, such as MS, over a spinal cord tumor.
Box 17-7 Spinal Cord Enlargement
OTHER INFECTIONS
Astrocytoma
These tumors may produce mild scoliosis, widen the interpediculate distance, and cause vertebral scalloping but less than ependymomas. They are isointense to low intensity on T1WI and high intensity on T2WI. The average length of involvement is seven body segments. They have an associated cystic component in 60% of cases (usually tumoral with small irregular or eccentric morphology), which may be appreciated on T1WI as hypointensity (Fig. 17-19). Hemorrhage is uncommon. Syrinx is common in the pilocytic variety. Enhancement is the general rule, although it may be uneven compared with the intense enhancement of ependymoma. Malignant potential is generally less than that of brain astrocytomas. Exophytic components are sometimes present. The size of the tumor does not necessarily reflect its malignant potential (repeatedly we see that size is overrated). These tumors are associated with neurofibromatosis type 1 (NF-1), infiltrating and not generally resectable.
Ependymoma
They appear isointense to hypointense with respect to the cord on T1WI and may have a multinodular high signal picture on T2WI that occupies the whole width of the cord. Commonly there is edema surrounding the tumor. Ependymomas have a propensity to hemorrhage, with hypointense rims (“cap sign”) on T2WI, that histopathologically represent residual hemosiderin from hemorrhage. Ependymomas may cause subarachnoid hemorrhage. Hemosiderosis has also been reported to result from ependymoma. Enhancement is common, and the tumor may have sharply defined, intensely enhancing margins. They may be associated with extensive cyst formation (in up to 84% of ependymomas; mostly nontumoral cysts but tumoral cysts may also occur), which does not usually enhance. Other associated radiographic findings include scoliosis, canal widening with vertebral body scalloping, pedicle erosion, and laminar thinning (Fig. 17-20). Diffusion tensor imaging has been utilized to distinguish ependymoma, which displaces outwardly but does not infiltrate white matter tracts, from astrocytomas that disrupt the descending fibers.
Myxopapillary ependymoma (13% of all spinal ependymomas) may affect the filum terminale with extension into the conus and the subcutaneous sacrococcygeal region. The mean age at diagnosis is 35 years and the lesion is more common in males. The presentation is of low back pain, leg pain, lower extremity weakness, and bladder dysfunction. These lesions are multilobulated, usually encapsulated, mucin-containing tumors that may hemorrhage and calcify. On MR they are usually isointense on T1WI, high intensity on T2WI, and enhance (see Fig. 17-20). However, as a result of calcification or hemorrhage they may be high or low intensity on T1WI and T2WI. They can grow out neural foramina and simulate neurogenic tumors.
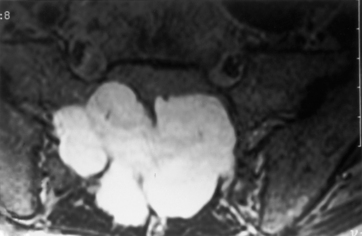
Hemangioblastoma
Hemangioblastomas are vascular lesions that may involve the cervical and thoracic spinal cord. They are the third most common primary intramedullary spinal neoplasm (1% to 7% of all spinal cord neoplasms). The tumor is composed of a dense network of capillary and sinus channels. They diffusely widen the spinal cord and may have both a cystic and solid component, with the solid component enhancing intensely. These tumors are associated with considerable edema (Fig. 17-21). Hemangioblastomas may be solid in 25% of cases.
Spinal cord hemangioblastomas may present as isolated lesions (80%), but one third of cases are associated with von Hippel-Lindau disease and are multiple (see Chapter 9). Von Hippel-Lindau disease has been isolated to a germline mutation of the VHL gene on chromosome 3. Hemangioblastomas are more commonly thoracic than cervical, and 43% have associated cysts.
Intradural Metastatic Disease
Metastases can deposit on the dura, pia-arachnoid region, and rarely in the cord itself (Fig. 17-22 ; see Fig. 17-18). The incidence of leptomeningeal and intramedullary metastases (up to 3% of patients with metastatic disease) is increasing as patients with cancer live longer. Patients may have nonspecific symptoms (headache, back pain) or focal neurologic symptoms. Intramedullary lesions result from tumor growth along the Virchow-Robin spaces because of CSF spread. Spinal cord metastases may also occur as a result of hematogenous dissemination. Intramedullary metastases enlarge the cord, are associated with edema, and enhance (see Fig. 17-18). The most common locations are the thoracic and cervical cord. They are usually solitary lesions involving two to three vertebral body segments. Box 17-8 lists lesions that commonly metastasize to the spinal cord or its coverings.
Cerebrospinal fluid spread of tumor in the leptomeninges is most often diagnosed by enhanced MR, but findings on lumbar puncture in carcinomatous meningitis are abnormal, with positive cytology on serial punctures in 90% of cases. MR reveals enhancement of the metastatic nodules, producing an irregular cord margin rather than its usual smooth contour. The entire subarachnoid space can enhance (sugar-coated appearance), and tumor nodules can be seen on nerve roots or in the cauda equina (Christmas balls). Pial metastases reveal linear enhancement on the surface of the cord (see Fig. 17-22A); however, this can be observed in nonmalignant disease, including sarcoidosis and infection. The CSF on T1WI can be more intense than normal, and the conspicuity between CSF and cord may be diminished. Cerebral MR is necessary in this situation because leptomeningeal metastases may be the result of CSF seeding from brain parenchymal metastases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree