Name
SST1
SST2
SST3
SST4
SST5
Ga-DOTATATE
>10,000
0.2 ± 0.04
>10,000
300 ± 140
377 ± 18
Ga-DOTATOC
>10,000
2.5 ± 0.5
613 ± 140
>1,000
73 ± 21
Ga-DOTANOC
>10,000
1.9 ± 0.4
40 ± 5.8
260 ± 74
7.2 ± 1.6
Performance of the three 68Ga-based PET tracers has recently been reviewed [17]. For 68Ga-DOTATATE, sensitivity on a patient basis was reported to be 72–96 % in 6 smaller studies including between 18 and 38 patients (total of 144 patients) [18–22]. The study with the median sensitivity reported 93 % (CI, 70–99 %; 20 patients) [21]. For 68Ga-DOTATOC, sensitivity on a patient basis was found to be 92–100 % in 6 small- or medium-sized studies including between 8 and 84 patients (total of 211 patients) [23–28]. The largest study with 84 patients reported a sensitivity of 97 % (CI, 90–100 %) [26]. Finally, for 68Ga-DOTANOC, patient-based sensitivity was reported to be 68–100 % from 9 studies including 11–1,239 patients (total of 1,677 patients) [21, 22, 29–34]. The study with 1,239 patients reported a sensitivity of 92 % (CI, 90–94 %) [34].
Taking into consideration that the number of patients as well as the composition of the groups evaluated with the different 68Ga-based tracers varied, it seems fair to conclude that no major differences have been documented especially since the populations studied varied between the investigations. So far, only three studies have compared these tracers pair-wise on a head-to-head basis [21, 22, 35]. In all these studies, comparisons were undertaken lesion based rather than patients based since only minor differences were expected and since the superiority of one tracer could be ability to detect more lesions. Two studies compared 68Ga-DOTATATE with 68Ga-DOTANOC [21, 22], and one compared 68Ga-DOTATATE with 68Ga-DOTATOC [35]. Although minor differences were reported, many were nonsignificant on a patient basis, and considering clinical relevance in our view, there were no major differences favoring use of one of these three tracers. Local availability, experience, and wish to theranostic pairing, e.g., use of 68Ga-DOTATATE before 177Lu-DOTATATE is to be used for PRRT, may decide which tracer to use. Absolute uptake of tracer between the tracers did vary although not systematically. Therefore, if comparing absolute values as SUV longitudinally, e.g., in therapy response monitoring, the same tracer has to be used.
Recently, another 68Ga-labeled PET somatostatin receptor PET tracer, 68Ga-DOTA-lantreotide (68Ga-DOTALAN), was introduced mainly as a companion diagnostic for 90Y-labeled lanreotide. Two studies compared 68Ga-DOTALAN on a head-to-head basis with 68Ga-DOTATOC [36] and 68Ga-DOTATATE [37], respectively. Both studies found substantially less lesions visualized with 68Ga-DOTALAN, questioning the usefulness of this tracer as a stand-alone imaging agent but not necessarily as a companion diagnostics.
9.5.2 64Cu-DOTATATE
Recently we introduced the somatostatin receptor PET tracer 64Cu-DOTATATE for human use [38]. Theoretical advantages compared to 68Ga-labeled tracers include the longer half-life of 64Cu (13 h) compared to 68Ga (68 min) allowing for delayed imaging for up to 24 h. Indeed, we showed that imaging after 3 h rather than 1 h with 64Cu-DOTATATE seems optimal since most kidney activity is then cleared. Imaging with 68Ga-tracers at 3 h would not be possible due to the short half-life. Also, the positron range, which is the distance a positron travels from emission until it is annihilated and sends out two photons, differs between the radionuclides. Since the PET scanner detects the coincident photons and not where the emission took place, which is really what should be visualized, the image is off focus (blurred). For 64Cu the average positron range is only 1 mm whereas it is 4 mm for 68Ga. This translates into better resolution and potentially better detection of small foci using 64Cu as radionuclide. Finally, the long half-life and a shelf life of >24 h of 64Cu-DOTATATE allow us to produce the tracer and distribute it if wanted. Finally, logistically 68Ga needs a radiochemist to be on standby, as doses need to be produced in close relation to when needed. In contrast, 64Cu-DOTATATE is produced as one batch for use throughout the day and the next morning.
In our first-in-human study of 14 patients where we performed a head-to-head comparison with 111In-DTPA-octreotide [38], in 6 of these 14 patients, additional lesions were found using 64Cu-DOTATATE compared to 111In-DTPA-octreotide, and in 5 patients these were in organ systems not previously known as metastatic sites. After 18 months of follow-up, all additional lesions seen on PET could be confirmed as true positive. Based on the first 100 prospectively enrolled patients, we found a sensitivity of 91 % for 64Cu-DOTATATE. In 35 cases PET identified pathological foci in organs not identified with 111In-DTPA-octreotide. Of these, 31 were later confirmed to be true-positive lesions [39]. Taken together 64Cu-DOTATATE seems promising for clinical use. Currently, we are undertaking head-to-head comparison of 64Cu-DOTATATE with 68Ga-DOTATOC. Data are not yet available, but based on image quality better resolution as reflected by degree of detail is found using 64Cu-DOTATATE (Fig. 9.1).
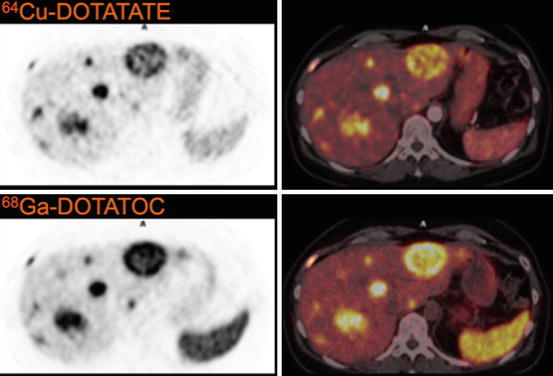

Fig. 9.1
Imaging of same NET liver lesions with 64Cu-DOTATATE and 68Ga-DOTATOC. Please note the greater detail in 64Cu-DOTATATE images, probably due to difference in positron range of 64Cu and 68Ga as well as the lower uptake in spleen (Reproduced with permission from Ref. [17])
9.6 Catecholaminergic and Serotonergic Pathways for Imaging of NETs
Neuroendocrine tumors have the ability for uptake of amine precursors by the L-type large neutral amino acid transport system (LAT) transporter and through further metabolism including decarboxylation to form catecholamines and serotonin.
9.6.1 18F-DOPA
The PET tracer and dopamine precursor 18F-L-dihydroxyphenylalanine (18F-DOPA) is taken up by neuroendocrine tumor cells by LAT, transported into the cytoplasm, and metabolized (decarboxylated) to dopamine, which is then transported into secretory vesicles by the vesicular monoamine transporter (VMAT). In the secretory vesicles dopamine is further metabolized to noradrenaline and adrenaline.
Early studies showed promising results for 18F-DOPA in GEP and bronchopulmonary NETs with sensitivities on a patient basis ranging from 65 to 96 % based on four studies with a total of 116 patients (meta-analysis sensitivity, 87 %; CI, 80–93 %) [9]. Even higher sensitivities were found for pheochromocytomas and paraganglioma that are, however, not the focus of this chapter. Performance in abdominal NETs was in general superior when compared to gamma camera-based somatostatin receptor scintigraphy. However, since PET-based somatostatin receptor imaging is superior as well, the question arises whether 18F-DOPA is better and valuable compared to the 68Ga-labeled somatostatin receptor PET tracers. Two studies with a total of 28 patients compared on a head-to-head basis 18F-DOPA with 68Ga-DOTANOC or 68Ga-DOTATOC, respectively. In both studies 68Ga-DOTANOC or 68Ga-DOTATOC visualized considerably more lesions than 18F-DOPA even in pheochromocytomas [29, 40]. One study with 25 patients compared 18F-DOPA with 68Ga-DOTATATE, and also here it was found that 68Ga-DOTATATE had a much higher sensitivity [20].
Taken together, although 18F-DOPA performs pretty well in GEP and bronchopulmonary NETs, when compared with modern PET tracers targeting somatostatin receptors, they are inferior. In general, it therefore seems that this tracer is not necessary for routine use if somatostatin receptor scintigraphy is performed by PET although we cannot rule out that in selected, difficult cases it may be of some value.
9.6.2 123/131I-MIBG
A reuptake mechanism exists at the catecholaminergic terminals for noradrenaline. This reuptake mechanism can be visualized using the false neurotransmitter metaiodobenzylguanidine (MIBG) which is also a substrate for the reuptake mechanism. MIBG can be labeled with radioiodine and is most commonly used for SPECT as 123I-MIBG or 131I-MIBG, where the latter may also be used for therapy due to concomitant beta-emission.
For imaging of GEP and bronchopulmonary NETs, the sensitivity of 123/131I-MIBG gamma camera imaging is somewhat disappointing based on two studies with a total of 125 patients: 63 % (CI, 54–72 %) [9]. For pheochromocytomas, neuroblastomas, and paragangliomas, the same meta-analysis found sensitivities of 79 % (9 studies; n = 161; CI, 68–82 %), 84 % (5 studies; n = 204; CI, 79–89 %), and 69 % (4 studies; n = 87; CI, 58–78 %), respectively. Again, the question arises whether 123/131I-MIBG imaging has any role when somatostatin receptor imaging and FDG-PET are available. In a recent prospective study, we therefore performed 111In-DTPA-octreotide SPECT, 123I-MIBG, and FDG-PET for head-to-head comparison in a total of 96 consecutive patients [12]. We found an overall sensitivity on a patient basis of 52 %, and 111In-DTPA-octreotide detected more than twice the number of lesions compared to 123I-MIBG. Of the 96 patients, 3 were 123I-MIBG positive and 111In-DTPA-octreotide negative. However, all of these were also FDG-PET positive. Accordingly, when somatostatin receptor scintigraphy and FDG-PET are available, we found no additional value of 123I-MIBG. Therefore, we doubt that there is any future role of 123I-MIBG imaging in NETs apart from companion diagnostics if 131I-MIBG treatment is planned. For this reason, we do no longer offer 123I-MIBG as a routine method for net at our department.
9.6.3 11C-5-HTP
The PET tracer and serotonin precursor 11C-5-hyroxytryptophan (11C-5-HTP) is transported into the neuroendocrine tumor cells via LAT, decarboxylated to serotonin, and transported into secretory vesicles by VMAT.
The PET tracer is labeled with 11C, which has a half-life of 20 min. This demands an on-site cyclotron, and that synthesis is made in close relation to the scan. Accordingly, the capacity of 11C-5-HTP PET scans is limited and only available in few centers. Maybe for this reason, also data on performance is somewhat limited. A total of 54 patients were studied in three different investigations [41–43], and when data were pooled a sensitivity of 87 % (CI, 75–95 %) was found [9]. One of these studies compared the performance with 18F-DOPA and 111In-DTPA-octreotide and found 18F-DOPA best for staging in carcinoids (n = 24), whereas 11C-5-HTP was best in pancreatic islet cell tumors (n = 23) [43]. In another study with 42 consecutive NET patients in comparison with 111In-DTPA-octreotide, 11C-5-HTP found more lesions in 58 % of patients, and it was concluded that 11C-5-HTP could be used as a universal imaging method for detection of NETs [42]. However, no other PET-based imaging was included in this study, and the superiority found was probably largely due to difference between SPECT and PET performance. Moreover, no studies until now have compared 11C-5-HTP with PET-based somatostatin receptor scintigraphy and FDG-PET. Accordingly, at present it is unsettled whether 11C-5-HTP would be the method of choice in unclear NET cases. One could speculate if combined somatostatin receptor PET and FDG-PET would not solve most of such cases in an easier way?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






