Occult Spinal Dysraphism
The term occult spinal dysraphism1 (OSD) refers to a group of disorders arising as a result of the malformation of midline dorsal neural, mesenchymal, and cutaneous ectodermal structures during embryogenesis. OSD was originally grouped by von Recklinghausen2 with spina bifida aperta because they share defective lamination of the posterior arches and present as abnormalities of skin, fat, laminae, and neural tissue. Over the past two centuries, OSD has come to encompass diastematomyelia and split-cord malformation (SCM), meningocele manqué, dermal tract or sinus with and without intraspinal tumors, lipoma of the conus and/or filum, and other abnormalities of the filum.3,4
Although these conditions vary in their clinical features, embryopathology, and response to treatment, the clinical disorders that are categorized as OSD have four features in common: (1) The major causes of neurologic impairment in these patients are spinal cord tethering, neural compression, and myelodysplasia; (2) when no surgical treatment is instituted, patients may develop progressive neurologic deficits as a result of cord tethering, trauma, or compression; (3) progressive and evolving deficits are amenable to surgical intervention, whereas static deficits are not5–8; and (4) anatomical and postoperative clinical retethering occurs and requires that these patients be monitored for neurologic, urologic, and orthopedic deterioration, even after a successful operation.5,9
The clinical features that characterize OSD have been the subject of interest through the ages. The “faun’s tail” that commonly accompanies diastematomyelia, the pes cavus, and the calf atrophy may have been the genesis of the satyr of Greek mythology.2 Descriptions of SCMs, including the introduction of the term diastematomyelia with and without bony septum and fibrous bands, “all associated with greater or lesser degrees of spina bifida,” appeared in the late 19th century.10 The surgical significance of SCM had to await the reports of Marr and Uihlien 1944,11 and of Matson et al in 1950.12 The importance of congenital lipomas of the conus and filum was initially brought to attention by Bassett in 1950 in a report of patients who had lipomas of the conus and filum, presented with urinary incontinence due to neurogenic bladder, and recovered continence postoperatively. Walker and Bucy13 were the first to recognize the association between congenital dermal sinus, meningitis, and paralysis. Tethering of the spinal cord by the filum terminale as a cause of neurologic deterioration was a concept suspected by Garceau.14 Hoffman et al15 introduced the term tethered cord syndrome to refer to this entity. Subsequently, its use was expanded to include restricted cord movement with any and all etiologies.16
25.1 Epidemiology
The incidence of OSD in the general population is not known. Unlike the diagnosis of open defects, which are clinically obvious, that of closed defects requires awareness of the significance of cutaneous markers and often subtle neurologic deficits and musculoskeletal deformity. There is little information regarding the risk factors predisposing to nonsyndromic closed spinal dysraphic states. The observation that open and closed neural tube defects may be genetically related17 suggests that at least some risk factors may apply to both types of spinal malformations. Studying 364 siblings of 207 patients with all forms of OSD, Carter et al found a 4% incidence of myelomeningocele or anencephaly in the series. Patients with OSD and families with caudal regression syndrome may share risk factors, in particular, maternal diabetes.18,19
The incidence of anomalies associated with cord tethering, including imperforate anus, have been shown to decrease with the periconceptual consumption of folic acid.20 However, dietary folic acid supplementation has not been shown to decrease the incidence of lipomyelomeningocele in Nova Scotia.21
25.2 Pathology and Pathogenesis
25.2.1 Intradural Lesions
Tight Filum Terminale
An abnormally tight filum terminale causes caudal traction on the spinal cord.4,14,15 The diagnosis is suspected on clinical grounds and confirmed when the conus position is abnormally low, or is in a normal position (above L2),15,22,23 by the presence of fat in the filum (“fatty filum” or “filum lipoma”)24,25 and/or a filum diameter greater than 2 mm (“thick filum”) (▶ Fig. 25.1). Although such findings (low conus, fatty filum, and thick filum) may be seen occasionally in isolation in a normal patient, the combination of two or more factors, coupled with the patient’s clinical status and/or the presence of bony dysraphism, should significantly increase the likelihood of finding a symptomatic tethered spinal cord.26
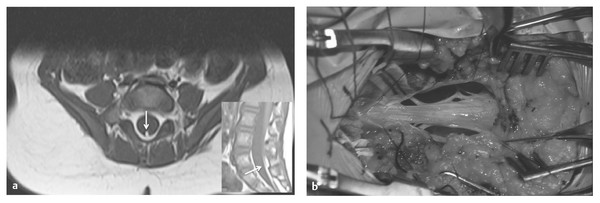
Fig. 25.1 (a) Transverse and midsagittal magnetic resonance images of a small infant with a tethered spinal cord resulting from a tight filum terminale. The conus is abnormally low (below L3), and the filum is enlarged and infiltrated with fat (arrows). (b) Thick, tethering filum terminale. Roots ascend to reach their exit foramina.
Split-Cord Malformation (Diastematomyelia)
SCMs can be divided into two main types, classically termed diastematomyelia10 and diplomyelia, and were previously thought to have different embryologic origins. In an effort to provide a classification scheme with more consistent and clinically relevant terminology, Pang,27,28 building on the theories of Bremer29 and the classification used by James and Lassman,4 suggested the use of SCM type 1 and SCM type 2. Type 1 SCM consists of two hemicords separated by an osteocartilaginous median septum, each housed in a separate dural sheath (▶ Fig. 25.2a). Type 2 SCM consists of two hemicords contained within the same dural envelope and separated by a fibrous septum (▶ Fig. 25.2b). Both SCM types are thought to be caused by an abnormal, persistent neurenteric canal between the yolk sac and amnion. This fistula later splits the neural canal and notochord by forming an endomesenchymal tract. The persistence of parts of the tract, the entrapment of different structures within it, or both, explain the subsequent development of associated malformations. Endodermal remnants predispose to the formation of neurenteric cysts and intestinal duplication, the lack of skin closure dorsal to the tract causes a dermal sinus tract that may form a dermoid cyst, and the entrapment of neural crest elements within the endomesenchymal tract allows the formation of paramedian nerve roots and meningocele manqué.28 A persistent endomesenchymal tract interfering with normal neurulation of the adjacent neural tube may explain the known association of SCM with open myelomeningocele.
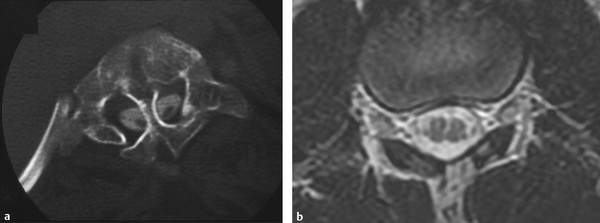
Fig. 25.2 (a) Computed tomographic myelogram of the upper lumbar spine. Dysmorphic bone is applied over the dorsal surface of the cord. A bony septum with a dural sleeve penetrates the split on this and adjacent images. (b) Axial magnetic resonance image of a type 2 split-cord malformation. The patient’s symptoms were caused by tethering bands arising from the medial aspect of each hemicord and fixing the cord dorsally. The bands are not visualized on this study.
Most if not all patients have tethering median septa, the residual of the neurenteric canal, that are not always visible on preoperative imaging studies. All lumbar SCMs have a low-lying conus and may have additional tethering lesions.27
Meningocele Manqué (Dorsal Bands)
Meningocele manqué (dorsal bands) is an occult spinal lesion that, as the term implies, is a meningocele that failed to develop (from the French manqué, “missed” or “failed”) (▶ Fig. 25.3). According to James and Lassman,4 who coined the term, an open meningocele “protrudes on the surface of the back and does not contain neural elements. But often, there are nerves that are adherent to the neck of the meningocele.” It is thought that a meningocele manqué is a meningocele that formed in the embryo, then underwent spontaneous healing or scarring. It is often described as a dorsal band with or without adherent nerve roots or dorsal root ganglia. These bands start in the intrathecal structures and extend up into the dura or lamina, thus possibly tethering the spinal cord. Histologically, these bands are composed of fibrous tissue that often contains meningeal elements. Most of these lesions are associated with other intradural lesions.30–33
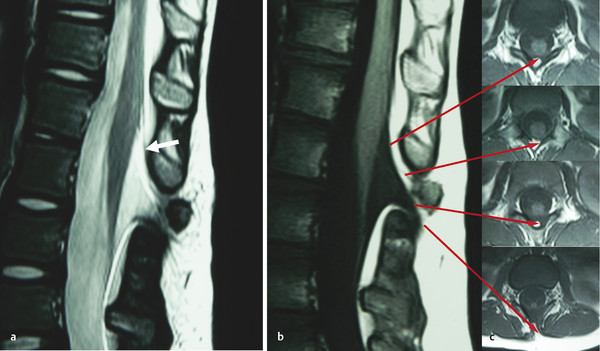
Fig. 25.3 (a) T2-weighted sagittal magnetic resonance (MR) image showing features of meningocele manqué, including a meningocele, tapered conus, dorsal tethering bands (arrow), and a rudimentary disk at level of the dysplastic lamina. (b) T1-weighted MR image of the same section as in (c) axial images. Adjacent axial images show a dorsal lipomatous stalk descending from the conus to exit the spinal canal through a bifid spinous process and passing into the subcutaneous fat (lowest image).
Dermal Sinus and Associated Tumors of Disordered Embryogenesis
Dermal sinus ▶ Fig. 25.4) and tumors of disordered embryogenesis result from incomplete separation of the neural ectoderm from the epithelial ectoderm (incomplete disjunction).34 Dermal sinuses occur most commonly in the midline at the lumbosacral junction but are known to occur anywhere in the midline from the sacrum to the nasion and in rare situations may be off midline over either the spine or cranium. The sinus is lined with epithelium encased with dermis and fibroglial tissues. There may be hair protruding from the orifice of the sinus, itself often surrounded by avascular nevus. Most sinuses pass into the intradural space to end on the filum terminale or the conus. It is rare that they end in the subcutaneous tissues or extradural space.35

Fig. 25.4 (a) Dermal sinus and tract passing through dura to attach to the dorsal surface of the conus. (b) Dermal sinus tract is easily separated from the cauda equina and filum. (c) Dermoid tumor in the dermal sinus tract. (d) Dermoid tumor containing desquamated epithelium and hair.
Tumors may occur either independently or in association with a dermal sinus tract. Dermoid tumors occur in 80% of patients and contain elements from two germ layers (e.g., skin, hair, sweat, and sebaceous glands). Epidermoid tumors (20%) contain only desquamated cells from the epidermal layer. Intraspinal teratomas, which are more unusual, contain elements from all three germ layers. Malignancy is rare. Dermoid tumors may present as a midline subcutaneous mass, may be embedded in the central canal of the spinal cord, or may appear anywhere between these two extremes. The wall of the cyst contains the epithelial elements that are associated with regrowth of the cyst; thus, the focus of the resection must be to remove all epithelial remnants if possible.4,34
Terminal Syrinx
A terminal syrinx (▶ Fig. 25.530,36) is a cystic dilatation of the lower third of the spinal cord. It generally occurs cephalad to other OSD defects (tight filum terminale associated with an anorectal anomaly, meningocele manqué, diastematomyelia, and lipoma of the conus) and less frequently with other spinal cord anomalies.30 It is present in about one-third of cases of OSD evaluated by magnetic resonance (MR) imaging, and two-thirds of these are large and likely to cause pain, motor weakness, and bowel and bladder deficits. Terminal syrinx should be suspected as a cause of delayed deterioration in function in a patient with OSD.
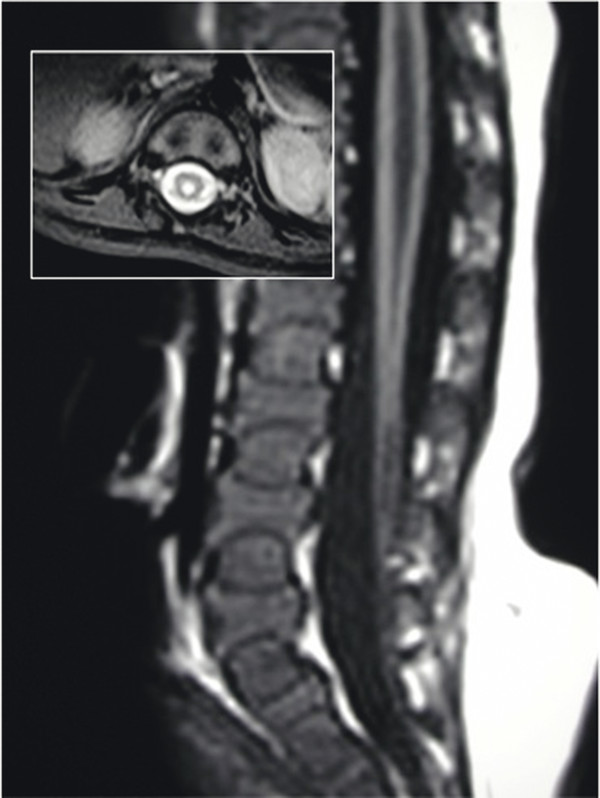
Fig. 25.5 Magnetic resonance image of the lumbar spine of an infant with a moderate-size syrinx and a conus tethered by a thickened filum. Insert shows the extent of the syrinx in the transverse plane.
A myelocystocele (▶ Fig. 25.6) is probably a severe form of terminal syrinx that balloons out to form a large terminal cyst that tethers the spinal cord. The cyst sits in a large cavity that consists of an arachnoid-lined meningocele.37–39 This meningocele is in continuity with the subarachnoid space and is associated with dorsal bony dysraphism. The myelocystocele may be continuous with a syrinx and may be associated with a lipomatous mass, hence the terms lipomyelocystocele and cystolipomyelomeningocele.40 For unknown reasons, myelocystoceles have been strongly associated with the teratogenic agent retinoic acid, as well as with the OEIS (omphalocele, cloacal exstrophy, imperforate anus, spinal anomalies) syndrome.41,42
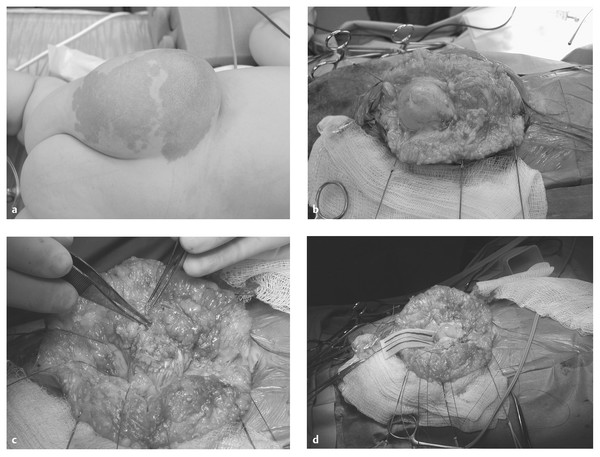
Fig. 25.6 (a) Lipomyelocystocele. Spinal ultrasound demonstrated several cerebrospinal fluid (CSF)–containing spaces. The terminal myelocystocele was recognized with gentle ballottement of the mass and observation of expansion of the central canal rostral to the lesion. The other CSF space was presumed to be a meningocele. (b) Exposure of the meningocele. (c) Meningocele opened. Lateral aspect of the myelocystocele at the lipoma–root interface clearly seen. (d) Meningocele and myelocystocele opened. Central canal in view.
25.3 Pathophysiology
Intradural dysraphic lesions cause neurologic deficits in several interrelated ways: (1) They may be due to abnormal formation of the neural structures during intrauterine development; (2) lesions such as a lipoma of the conus or tumors of disordered embryogenesis may compress or expand within the conus medullaris; and (3) these lesions may cause traction on the radicular and medullary vasculature, spinal cord, and nerve roots by tethering these structures to surrounding spinal canal, dura, or extradural tissues. Such tethering is thought to interfere with normal, growth-related cord, root, and vascular motion, resulting in excessive tension within the cord.16,43,44 Tethering may cause traction on the conus medullaris or cord caudally (e.g., tight filum terminale and lipomyelomeningocele); dorsally (e.g., meningocele manqué, dorsal band, and dermal sinus tract); ventrally (e.g., ventral band, diastematomyelia, and neurenteric cyst); or in several directions simultaneously (varieties of complex lipoma of the conus). In addition, neurologic impairment may be due to meningitis or abscess complicating a dermal sinus or to hemorrhage into a neurenteric cyst.
Tethering is thought to be a major contributor to the deterioration in function noted over time in patients with OSD.16,45–47 Experimental evidence has shown that traction causes ischemic changes to the spinal cord.16,43–46 Repetitive flexion and extension of the spine and other sudden violent movements of the body are thought to cause ischemia to the tethered spinal cord as well as direct trauma at the point of fixation.16,48,49 Repetitive motion of the cord and roots, driven by cerebral spinal fluid (CSF) pressure and flow changes, is also likely to be contributory to cord injury.50 Similarly, spinal cord traction is thought to worsen with growth of the spinal column during childhood, thus explaining the progression of the neurologic deficit over time. Traction on the spinal cord is seen to cause maximal cord elongation in the lumbar region, with less physical damage or “stretchability” in the thoracic and cervical areas.16,48 This finding fits the usual clinical scenario of maximum lumbosacral cord dysfunction with the tethered cord syndrome. MR imaging of cord motion has confirmed that cord motion is decreased in patients with tethering and has suggested that the prognosis for neurologic recovery is worse in patients who had markedly decreased cord motion preoperatively.51–53
25.4 Presentation
25.4.1 Signs and Symptoms
Patients with occult spinal dysraphism (OSD) may present with a combination of cutaneous, orthopedic, urologic, and neurologic signs and symptoms. The box ▶ “Signs and Symptoms of OSD” lists the patterns of clinical presentation and manifestations that suggest the diagnosis of OSD.4,54 As the knowledge of OSD broadens among practitioners, many cases will be recognized in infancy and childhood based on the clinical syndrome of cutaneous anomaly, subtle or progressive neurologic deficits including urinary incontinence, and musculoskeletal asymmetry.
Patterns of Clinical Presentation
Asymptomatic infant with cutaneous marker
Asymptomatic infant or child with caudal regression syndrome
Symptomatic child with incontinence/evolving neurologic or orthopedic anomalies.
Symptomatic child with secondary incontinence and a normal filum on MR imaging (occult tethered cord syndrome).
25.4.2 Clinical Context
The clinical context in which the patient presents can be the same for a number of pathologic forms of OSD. Although certain cutaneous markers and skeletal deformities are relatively specific for underlying spinal cord abnormalities (see box ▶ “Signs and Symptoms of OSD”), many are not and are defined only after spinal MR imaging or ultrasound. In general, the clinical presentation falls into one of the following categories:
Asymptomatic infant with cutaneous marker
Asymptomatic infant or child with caudal regression syndrome
Symptomatic child with incontinence/evolving neurologic or orthopedic anomalies
Symptomatic child with secondary incontinence and a normal filum on MR imaging (occult tethered cord syndrome)
Signs and Symptoms of OSD
Cutaneous stigmata
Hypertrichosis
Orthopedic deformities
Foot and leg deformities and asymmetry
Scoliosis
Urologic problems
Neurogenic bladder
Urinary tract infections
Incontinence
Neurologic symptoms and signs
Infants
Decreased spontaneous leg movement
Absent reflexes
Leg atrophy hidden by baby fat
Foot asymmetry
Toddlers
Development delay (walking)
Abnormal gait
Older children
Asymmetric motor and sensory dysfunction
Painless foot burns
Upper motor neuron signs (hyperreflexia)
Back and leg pain
Young adults
Back and leg pain, either chronically or acutely after trauma or hyperflexion
Spasticity and hyperreflexia
Any age
Meningitis
Paraplegia
Asymptomatic Infant with Cutaneous Marker
The incidence of significant cutaneous lesions of the craniospinal axis in the general population of neonates was found to be 3% in a large prospective study,55 whereas in patients with OSD, the incidence approaches 80%,4,7 and it is common for two or more cutaneous lesions to coexist.56 Cutaneous anomalies occur in the midline of the back, are above the level of the coccyx, and commonly overlie the spinal lesion. In general, the complexity of the cutaneous lesion reflects the degree of developmental anomaly of the underlying neural structures (see box ▶ “Signs and Symptoms of OSD”). The cutaneous lesions associated with abnormalities of the filum, tethering bands, tracts, and neurenteric cysts include dimple, subcutaneous lipoma, dermal appendages, deviated or forked intergluteal cleft, vascular nevus, and/or pigmented nevus.57
In hypertrichosis, tufts of hair may be sparse but more commonly are profuse, earning the name “faun’s tail” or “horse’s tail” (▶ Fig. 25.7a,b).2,4 There is a strong association between hair tufts and SCM, especially the form with a median bony septum (type 1). In the series of James and Lassman, hypertrichosis, especially the profuse form, occurred in 67% of cases of diastematomyelia with a median septum and in 33% of cases without a septum. In the same series, hypertrichosis also occurred in 24% of patients with spinal anomalies other than diastematomyelia.4
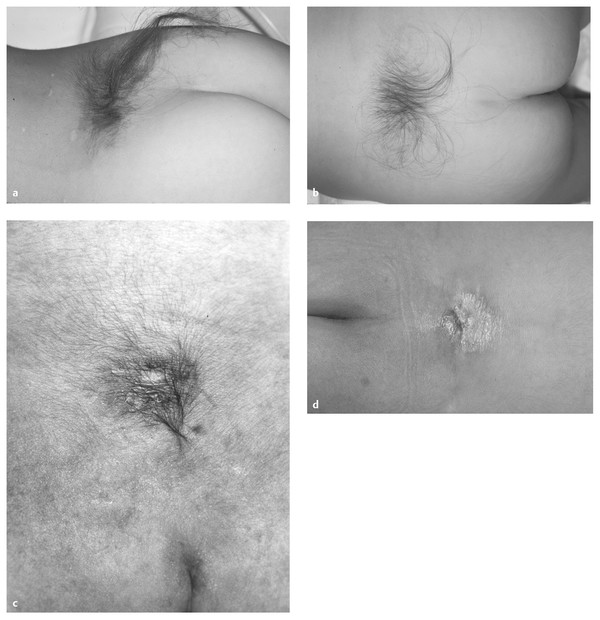
Fig. 25.7 (a,b) Faun’s tail. Luxuriant hair reminiscent of the tail of a satyr. (c) Infant with focal hirsutism superimposed on a background of flat capillary hemangioma. The central aspect of the lesion is composed of thin, atrophic skin and is exquisitely sensitive to touch. From the base of the lesion, strands of tissue extended to the dorsal surface of the spinal cord, which they tethered. (d) Aplasia cutis. The cigarette burn of old. This may mark a dermal tract without sinus, or meningocele manqué.
Atretic meningocele is a midline area that represents a meningocele that was present in fetal life and has subsequently atrophied or partially healed (▶ Fig. 25.7c,d). The skin can be quite thin, with the bluish hue of CSF underneath. Bands may connect the base of the skin defect to the dorsal surface of the spinal cord, and the lesion can be quite sensitive to touch, even in neonates. Aplasia cutis is often a small midline circular defect, in the past likened to a cigarette burn that commonly overlies a tethering dermal or fibrous tract. Atretic meningocele can be differentiated from aplasia cutis by the absence of the bluish hue in aplasia and the presence of a tract on imaging.
A dermal sinus is an opening in the skin that may connect to a subcutaneous tract lined by epithelium that can be traced to the dura or spinal cord (▶ Fig. 25.8). The orifice is commonly raised, with one or more stiff hairs sprouting, and it may have at its base a cutaneous hemangioma or nevus. Intradurally, it may be associated with any of the other major dysraphic anomalies, and half of the tracts that enter the spinal canal end in a dermoid or other inclusion cyst or tumor.34 A dermal sinus is especially significant among the cutaneous stigmata of OSD because in addition to its role as a sign of intradural pathology, it provides access for bacterial contamination that can result in local infection, meningitis, and/or intramedullary abscess.58 Such infections do not always have cutaneous manifestations (i.e., redness, swelling around the sinus opening). A dermal sinus should be distinguished from the common sacrococcygeal or dermal pit. The latter is always located in the intergluteal fold directly over the tip of the coccyx. Coccygeal dermal pits are not associated with OSD, and when seen in isolation, they require neither surgery nor radiographic evaluation.59
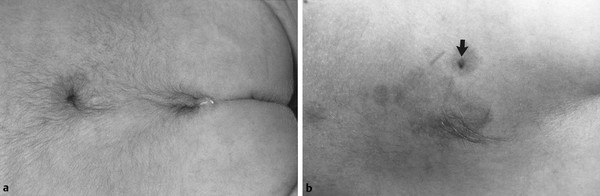
Fig. 25.8 (a) Dermal sinus—elevated orifice, stiff hair, and basal hemangioma. (b) Lumbar region of an infant with three cutaneous signatures of occult spinal dysraphism: dermal sinus (arrow), small area of focal hirsutism, and a flat capillary hemangioma under both of the other lesions. At operation, the dermal sinus was found to penetrate the dura and ascend to the conus; there was a short segment of diastematomyelia without a median septum, and the filum was enlarged and infiltrated with fat.
Dermal appendages can take many forms, some complex and reminiscent of abortive twinning60,61 (▶ Fig. 25.9). More commonly, they are simple, skin-covered mesenchyme-containing structures that resemble a tail. A pseudotail is any cylindrical outgrowth from the lumbosacral area (▶ Fig. 25.10); it is usually short and stumplike and may contain fat, cartilage, or other organ-specific tissue, such as embryonic kidney.62,63 In contrast, a true human tail is a remnant of an embryonic structure containing vertebrae, notochord, and spinal cord, as well as a sacral artery and vein; it may contain muscle in addition to adipose and connective tissues and may be curved, pigmented, or covered by hair; it is often “capable of spontaneous or reflex motion.”62
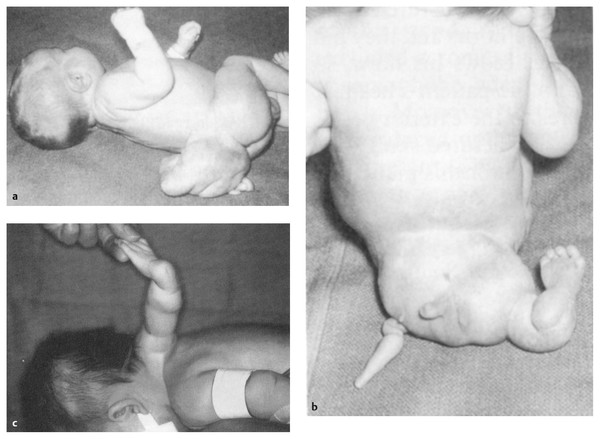
Fig. 25.9 (a–c) Examples of complex dermal and mesenchymal appendages.
([a] and [b] From Parkinson D. Accessory limbs and spinal dysraphism. J Neurosurg 1991;75:498–499.61 [c] From Humphreys R, Manwaring KH, Carroll N. Accessory arm—dysraphsim of disparity. J Neurosurg1991;74: 297–300.60 Reprinted with permission.)
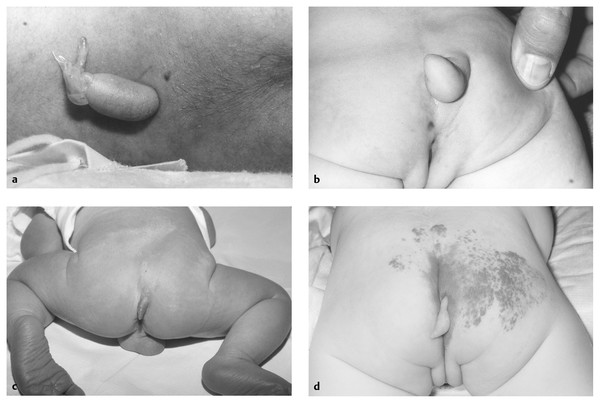
Fig. 25.10 (a) Small midlumbar appendage is easily seen. It has a very narrow neck and was easily excised, leaving a small scar. Imaging was performed, and a tethered spinal cord was clearly noted. The cord was untethered prophylactically, and the patient remains neurologically intact. (b) Another appendage, this one associated with rectal stenosis. Again, this is a cutaneous signature of occult spinal dysraphism. (c) Dermal appendage marking a lipoma of the filum. (d) Dermal appendage associated with terminal lipoma of the conus.
An asymmetric or forked gluteal cleft is often associated with a capillary hemangioma or dermal appendage.
The presence of a capillary hemangioma, either flat or raised (strawberry), in the midline over the spine raises the suggestion of an underlying dysraphic defect,4,64 in particular when it is associated with other cutaneous anomalies (lipoma, dimple, asymmetric gluteal cleft). The incidence of underlying dysraphism is low with an isolated lumbosacral midline strawberry hemangioma65 but is higher in the presence of a flat capillary hemangioma66 (▶ Fig. 25.11). Like a capillary hemangioma, a pigmented nevus is rarely a marker of OSD when it occurs in isolation, and mongolian spots are not markers of underlying spinal dysraphism.
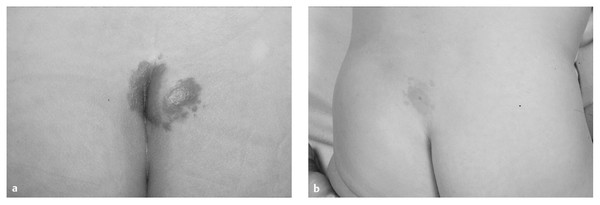
Fig. 25.11 (a) Intergluteal hemangioma marking lipoma of the filum. (b) Capillary hemangioma and dermal sinus.
These skin lesions are present at birth and often lead to an ultrasound or MR examination to detect intraspinal anomalies. The detection rate for tethered cord abnormalities varies in individual series, some reporting a high sensitivity and others low.57 Given the concerns regarding general anesthesia67 in this age group, it is the practice of this author to defer general anesthesia for MR evaluation in an asymptomatic infant until after 1 year of age if the ultrasound assessment is nondiagnostic.
Asymptomatic Infant or Child with Caudal Regression Syndrome
Caudal agenesis68–71 comprises a spectrum of sporadic congenital malformations arising from the caudal embryo. The range of hindgut and urogenital malformations69,70 that occur secondary to caudal agenesis includestenotic or imperforate anus; rectovaginal or rectourethral fistula; persistent cloaca resulting in a common urinary, genital, and intestinal outflow structure; and cloacal exstrophy. The complex developmental anomalies shown to be associated with spinal cord tethering include the following: (1) OEIS syndrome41 (omphalocele, cloacal exstrophy, imperforate anus, and spinal anomalies); (2) VATER syndrome72 (vertebral anomalies, anal imperforation, tracheoesophageal fistula, and renal radial anomalies); (3) VACTERL association (vertebral defects, anal atresia, cardiovascular anomalies, tracheoesophageal fistulas, renal anomalies, and limb defects, most often of the radius); and (4) the Currarino triad (anorectal malformation, sacral anomalies, and presacral masses). The more complex the developmental anomaly, the more frequently OSD is found,73–77 with incidence rates ranging from 15% in patients with an isolated imperforate anus (high or low)78 to 60% in those with the VACTERL association.79 Filum lipoma, thickened filum, lipomyelomeningocele, ependymal cysts, and myelomeningocele have been reported as the common tethering pathologies in these entities.
The role of the tethered cord in the functional impairment that these patients exhibit is not usually clear because of the pelvic floor, sphincter, and muscular malformations that directly affect urinary and bowel continence. Cord untethering is indicated for patients showing neurologic or motor deterioration; however, prophylactic untethering in the hope of improving continence is not necessarily indicated.80
Infants with caudal regression syndrome have typical clinical findings.70 A narrow pelvis results from the absence of vertebral segments and is accompanied by flattening of the buttocks, shortening of the intergluteal cleft, and prominence of the iliac crests. The missing coccyx and sacral elements may be obvious on palpation of the spine. Disturbance of lower extremity motor function is often accompanied by the relative preservation of sensory function. Treatment of the gastrointestinal and urogenital anomalies takes priority because of the possibility of life-threatening complications if they are left untreated.25
Symptomatic Child with Incontinence/Evolving Neurologic or Orthopedic Anomalies
No characteristic neurologic symptoms or signs occur in a patient with closed spinal dysraphism. The presentations range from back or leg pain, to subtle bladder disturbances detected with urodynamics, to a mild sensory disturbance in one foot, to severe motor deficits and resultant leg atrophy and deformity. The neurologic deficits are typically asymmetric, regardless of the type or location of the spinal lesion.4,9 Before a child begins to walk, it is often difficult to detect muscular weakness. Often, the deficit is mild and evidenced only by a slight asymmetry of the feet (possibly indicating long-standing motor weakness) or a predisposition to painless foot ulcers. The difficulty with diagnosis is especially true in newborns, in whom a thorough neurologic examination is particularly challenging and in whom lower extremity changes become more evident over time as a consequence of the effects of muscle imbalance, weight bearing, and gravity on developmentally malformed bone and joint structures.81 Even unilateral atrophy of the leg muscles in an infant may be hidden by the abundant subcutaneous fat, and decreased spontaneous movements or an unusual posture of one extremity or asymmetric use may be an indication of subtle motor weakness.45 Delayed or asymmetric ambulation in an infant may be the initial complaint.
Rarely, infants with terminal myelocystocele may deteriorate quickly. The deterioration may be due to an increase in CSF volume within a terminal myelocystocele and syrinx82 and/or displacement of CSF into a terminal syrinx, with cord distention caused by supine positioning. Acute deterioration can also occur as a result of hyperflexion movement(s).
Unilateral or bilateral foot deformities4 are the most common deformities reflecting OSD and include club feet; valgus, varus, and cavovarus deformities; hallux varus; asymmetric toe size; and trophic foot ulceration. Anomalies may be subtle, especially in infancy, and may consist of an increase in the web space or a difference in the slope of the arch; these are best viewed by looking at the soles of both feet simultaneously. Buttock asymmetry with a lateral curve to the upper part of the gluteal crease is a subtle sign that can easily be overlooked. Spasticity, as well as analgesia-related trophic ulcers and fatigue fractures, may also be obvious in an older child.4,83,84 Because of underlying malformed bone and joints, a foot deformity may worsen after repair of a spinal anomaly.4,81
Patients harboring a dermal sinus usually present because the cutaneous marker is recognized. However, if this is missed or its significance is not appreciated, meningitis, often due to Staphylococcus aureus, Escherichia coli, or Proteus species, is the most common mode of presentation.13 Infection of an associated intraspinal dermoid tumor can result in acute paraplegia from either conus or cauda equina compression. As with other intradural tumors, spinal pain is a prominent feature.
Depending upon age, scoliosis occurs in up to 25% of patients with a tight filum terminale and 90% of patients with SCM. Factors that increase the likelihood of an intraspinal anomaly included the following: (1) thoracic curve convex to the left (23% of patients with left thoracic scoliosis had an intraspinal anomaly, versus 8% of patients with other curve patterns); (2) age younger than 11 years; (3) severe scoliotic curvature (the major curve averaged 57 degrees for the group with intraspinal anomalies and 28 degrees for the others); and (4) rapid progression of the curve (average of 28 degrees per year).85
Symptomatic Child with Secondary Incontinence and a Normal Filum on Magnetic Resonance Imaging (Occult Tethered Cord Syndrome)
Children who present with incontinence but no structural or functional urologic abnormality should be investigated for evidence of OSD, whether or not they have a cutaneous marker. The loss of urinary control may be primary when daytime and nighttime continence has never been acquired, or secondary when loss of control occurs after a period of normal daytime and nighttime continence. Several series have reported improvement in incontinence following section of a structurally normal filum associated with a normally positioned conus.86–89 At the present time, the clinical and urodynamic characteristics that predict continence following filum section are not clearly defined, and opinion among experts differs as to the role of untethering.90 It is hoped that a randomized clinical trial, currently in process, will provide clarity as to the operative indications in this clinical context. Until the results of this study are available, section of the normal filum for incontinence should be considered experimental.
25.5 Diagnostic Studies
25.5.1 Magnetic Resonance Imaging
Spinal MR imaging is the imaging procedure of choice to diagnose and follow patients with OSD. Its multiplanar imaging capability and the ability to visualize and differentiate neural tissue make it an ideal tool for congenital disorders of the spine. Complete imaging of the spinal cord is required to ensure the recognition of multiple “skip” lesions and the hindbrain herniation seen rarely in patients with lipomyelomeningocele and myelocystocele.91
T1-weighted images provide clear anatomical detail of the spinal cord and filum terminale, allowing visualization of the vertebral level of the conus; the presence or absence of fat within the cord, spinal canal, or filum; and the size of the filum. T2-weighted images allow the identification of spinal cord tumors such as dermoids and epidermoids. In addition, they highlight intramedullary fluid-containing structures such as syringes, myeloceles,92 and neurenteric cysts.
The assessment of the filum terminale in patients suspected of having tethered cord syndrome has been difficult, particularly in the situation in which the filum appears normal and the conus terminates at a normal level. With the improvements in imaging resolution, the ability to recognize abnormalities in the structure of the filum has also improved. The majority of patients with a thickened filum have fat and fibrovascular tissue on histologic examination.93–95 Fat in the filum is always found at the junction of the filum with the caudal dural tube and involves more of the rostral filum in individual patients. If the filum immediately caudal to the conus is free of fat for 13 mm or more, it appears that symptomatic cord tethering is unlikely.96
A variety of MR imaging sequences have been developed to evaluate cord motion, with the ultimate goal of being able to predict which lesions will require surgical untethering and to assess postoperatively the effectiveness of such procedures.51–53,97 Unfortunately, the goal of being able to use MR imaging to predict which patients will require untethering has not yet been achieved.
25.5.2 Ultrasonography
The role of ultrasonography in the evaluation or follow-up of patients with suspected closed spinal dysraphism is limited. Although it allows the operator to appreciate cord motion, its lack of spatial resolution limits its ability to define the causes of cord tethering. Despite these limitations and because of its low cost, ease of use, and minimal sedation requirements, ultrasonography has been used as a screening tool65,92,98,99 for asymptomatic infants with sacral dimples or isolated strawberry hemangiomas in whom the findings of a normal conus location, “normal” filum, and normal filum, conus, and root motion would allow the clinician to postpone MR imaging. In each of these situations, the probability of cord tethering/OSD is known or presumed to be low.26,95 However, when a diagnosis of OSD is suggested by clinical or other radiographic findings, or when the study is inadequate for technical reasons, spinal MR imaging is required, in particular to show subtle anatomical lesions. Ultrasonography has a specific role to play in the assessment of myelocystocele in infancy. The meningocele and myelocele fluid spaces are easily resolved, and the continuity of the myelocele with the central canal can be confirmed by gentle ballottement of the postsacral mass. This finding can be used to differentiate myelocystocele from lipoma of the conus.
25.5.3 Urodynamics
The objective monitoring of bladder function is often fraught with difficulty of interpretation, especially in infants and children, because cooperation with the study is an intricate component of the evaluation. The usefulness of urodynamic studies lies in the following: (1) the evaluation of patients who are being assessed for incontinence and whose imaging studies are normal,86,87,100 (2) the monitoring of neurologic function when a nonoperative approach has been elected for an infant or child with conus lipoma,5 and (3) the comparison of function before and after surgical untethering. A validated scoring system has been developed that describes urodynamic abnormalities in children.101
Significant derangements in filling capacity, pressure, compliance, and contractility are found in patients with OSD.102 Improvement has been demonstrated postoperatively.28,86,103 The use of urodynamic testing in the preoperative evaluation of patients and in the postoperative, long-term follow-up of selected patients is recommended.
25.6 Treatment
25.6.1 Natural History and Surgical Decision Making
Randomized studies comparing the natural history of OSD defects with clinical outcomes after operative intervention have been conducted infrequently.104,105 In part, this is due to the fact that historically, the disparate conditions grouped as OSD were viewed as having natural histories, surgical outcomes, and operative risks that were of similar magnitude regardless of the etiology. As a result, assumptions regarding the effects of cord tethering and the presumed long-term benefit of operation were generalized to a varied group of pathologic entities. Because the risk for operative neurologic injury is low in many entities, and because clinically apparent “neurologic deficit” is more common in older children, early operation, before the development of deficit, was recommended as a standard for all forms of OSD.
With the improvements in imaging and with further clinical experience, it has become apparent that the forms of OSD differ markedly in the ease and safety with which they can be surgically treated and in the effectiveness of surgery to address present and future clinical symptomatology (see box “Signs and Symptoms of OSD”). Surgery can address mass effect and in most cases cord tethering, but it does not address congenital myelodysplasia or predictably reverse established clinical defects.5,9,106–108
The underlying OSD state is of critical importance in determining (1) the evolution of symptoms and signs, including their clinical presentation; (2) the natural history, including that which is due to myelodysplasia, mass effect, and tethering; and (3) the safety and long-term efficacy of surgery. Both historical and contemporary surgical series demonstrate that patients‘ symptoms and signs are more likely to be recognized as they age.104,105,109,110 They also demonstrate that deficits (musculoskeletal, neurologic, and urologic) are more likely to improve or be reversed if appropriate treatment for the cause of deterioration is undertaken in a timely fashion following the onset of the deficits. Unfortunately, in many patients, it is not possible to determine whether a deficit is truly new and has a surgically treatable cause or whether it is due to congenital myelodysplasia that becomes evident as development progresses. The assumption that all children are normal and that deterioration is the result of surgically correctable lesions has led to the conclusion that patients with OSD, if untreated, will progressively lose neurologic function with time. This thesis also assumes that the risks of surgery are minimal and that the efficacy will be long-lived.
In general, simple OSD responds predictably to surgical intervention, whether for untethering (tract excision, excision of tethering bands, filum section) or tumor excision. As the operative procedures are in most situations straightforward, the intervention can be done with a low risk for neurologic injury.5,7,8,106,111–116 Unfortunately, this assumption is not true for patients with complex OSD and may not be true for those with simple forms that may retether symptomatically.117,118
25.6.2 Results of Surgical Treatment
The probability that a patient will recover from neurologic, urologic, and musculoskeletal symptoms varies with the cause of the symptoms and their duration. In general, a patient with fixed deficits or complete neurogenic bladder dysfunction does not recover as a result of operation, whereas a patient with progressive, evolving defects of musculoskeletal or neurologic function does recover to some degree. In the complex forms of OSD, a single untethering is not necessarily curative, and recurrence of similar or different symptoms is to be expected over the lifetime of the patient.5,7,9,106
Urologic dysfunction often improves after an OSD untethering operation.27,86,87,119–123
Back and leg pain is almost always relieved postoperatively.5,15,27,30,123,124
Scoliosis responds differentially in simple and complex OSD. In the former, scoliosis if minimal will resolve with untethering, but complex OSD, in particular SCM, may be associated with structural vertebral anomalies that lead to progression.
The intraoperative risk for neurologic impairment, most commonly to bladder function, is less than 10%, with acute risk to sensory and motor function less for initial untethering operations.
25.6.3 Preoperative Evaluation
Routine preoperative laboratory testing and MR imaging of the spine comprise the usual evaluation before surgery. Spinal computed tomography (CT) can be very helpful in determining the challenges to laminectomy and lateral resection to provide access. Urodynamic testing is useful in determining the preoperative urologic status.
25.6.4 Intraoperative Neurophysiologic Monitoring
The purpose of intraoperative electrophysiologic monitoring is to distinguish between functioning nerve roots and spinal cord tissue and nonfunctional tethering structures that can be sectioned, thus minimizing neural injury.125 Three main electrophysiologic techniques have been used in the operating room: (1) tibial and peroneal somatosensory evoked potentials (SSEPs), which theoretically detect excessive traction or lateral pressure on the conus; (2) pudendal sensory evoked potentials, which detect injury to the S2–S4 segments (which are very vulnerable to injury during an untethering procedure and are below the stimulation territories of the standard lower extremity SSEPs); and (3) free running electromyograms from lower extremity musculature including the anal sphincter and (4) uni- and bipolar stimulation of cord, roots, and/or tethering structures. Motor evoked potentials can be used to assess the effects of dissecion on the cord.45 Electrophysiologic improvement following surgical untethering can predate clinical recovery.126
25.6.5 Surgical Technique
A tight filum terminale is approached through a laminectomy, laminotomy, or laminoplasty extending from S1 to the mid sacrum (▶ Fig. 25.12) or through the L5–S1 interlaminar space. Exposure of the distal 2 or 3 cm of the subarachnoid space is all that is needed to allow adequate exposure.127 After the dural and arachnoidal opening, the filum is recognized by its posterior midline location, the bluish discoloration, the absence of nodes of Ranvier, its vasculature, and the fat commonly seen infiltrating the structure. The filum is separated from surrounding roots, then rotated to ensure that there are no nerve roots adhering to the undersurface. The filum is then coagulated and divided, first proximally, then distally, if a specimen is to be sent for histologic evaluation. The cut ends of the filum infrequently retether,117,118,128 making the long-term outcome excellent.
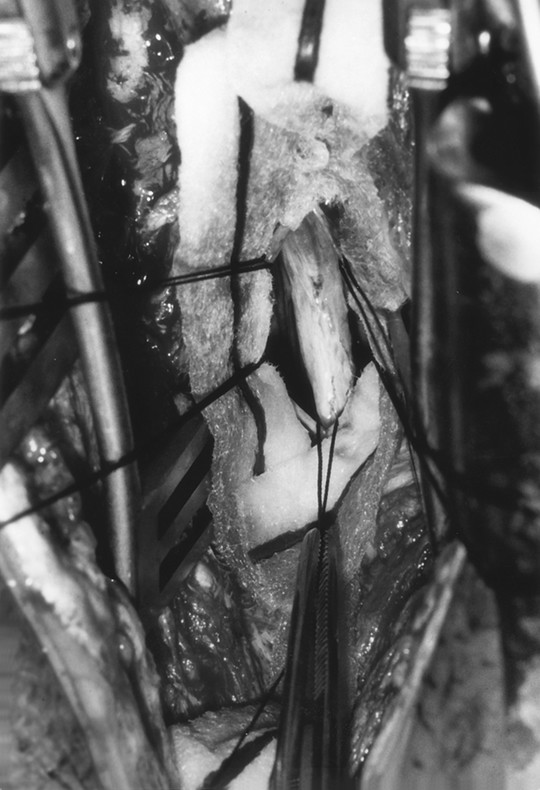
Fig. 25.12 Intradural view of a markedly thickened filum infiltrated with fat in a child presenting with back pain, scoliosis, and enuresis.









