Fig. 1
ChR2 and HaloR ChR2 senses blue light and open non-selective cation channel to induce depolarization. HaloR senses orange light and pump into chloride ion to induce hyperpolarization (Color figure online)
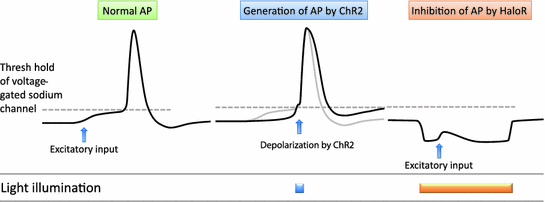
Fig. 2
Generation of action potential (AP) by ChR2 and inhibition of AP by HaloR. Depolarization by ChR2 reached to threshold of voltage-gated sodium channel generates AP. Hyperpolarization by HaloR prevents generation of AP
5 Activation of Orexin Neurons Using Optogenetics
In 2007, Dr. Deisseroth and Dr. de Lecea’s group at Stanford University applied optogenetics to freely behaving animals and successfully controlled sleep/wakefulness by targeting orexin neurons in the hypothalamus (Adamantidis et al. 2007). ChR2 was exclusively expressed in orexin neurons using a lentiviral vector. They generated mice in which orexin neurons exclusively expressed ChR2 by injecting a lentiviral vector containing the 3.1-kb upstream region of the mice prepro–orexin gene as a promoter. To confirm the function of ChR2 expression in orexin neurons, slice patch clamp recordings were performed. Blue light illumination induced depolarization of the ChR2-expressing orexin neurons. Both continuous and pulse light illumination was able to induce depolarization and increases in firing frequency.
A previous study showed that orexin neurons phasically fire during wakefulness and are almost silent during sleep (Mileykovskiy et al. 2005). To study whether artificial activation of orexin neurons induces wakefulness in mice, blue light illumination was applied in vivo in freely moving mice that displayed spontaneous sleep or wakefulness states. During spontaneous sleep, continuous or pulsative light illumination of greater than 5 Hz in frequency induced a transition to wakefulness after a delay of approximately 30 s from the initiation of light illumination. This effect was observed not only during NREM sleep but also during REM sleep. These facts suggest that artificial activation of orexin neuronal activity is sufficient to promote the initiation of arousal (Adamantidis et al. 2007). This study was the first successful report linking activation of specific types of neurons in mice to behavior by using optogenetics. In addition, this study established that optogenetics is a powerful tool to regulate neuronal activity and to control animal behaviors. Since this report, optogenetic approaches have been applied to the study of many neuronal types and various behaviors.
Another independent study used transgenic mice with orexin neurons specifically expressing melanopsin (OPN4) under the tetracycline gene expression control system (Tet-off) (Orexin–tTA; BitetO human OPN4/mCherry bigenic mice) (Tsunematsu et al. 2012). OPN4 is a blue light-driven Gq-coupling G protein-coupled receptor (GPCR) normally expressed in a subpopulation of retinal ganglion cells. Blue light illumination depolarizes OPN4-expressing neurons via activation of the Gq signal transduction cascade. The advantage of using OPN4 is that long-lasting depolarization can be induced using relatively weaker and shorter light pulses, as compared with ChR2 and GPCR signaling, which are slower and long-lasting. Additionally, using a transgenic mouse strain leads to reliable and reproducible expression of OPN4 in the orexin neurons compared to using the viral vector injection method. Short light pulse illumination evoked long lasting depolarization even after blue light cessation. Similar to the activation of orexin neurons using ChR2, blue light pulses induced the transition from NREM sleep to wakefulness in these mice.
6 Inhibition of Orexin Neurons Using Optogenetics
To inhibit orexin neuronal activity, an orange light-driven chloride pump, HaloR, was exclusively expressed in orexin neurons using transgenic methods. To express HaloR in orexin neurons, the 3.2-kb upstream sequence of the human prepro-orexin gene was used as a promoter to generate transgenic mice (orexin/HaloR transgenic mice). Immunohistochemical analyses revealed that more than 90 % of orexin neurons expressed HaloR in the orexin/HaloR mice (Fig. 3). At first, to confirm the function of HaloR expressed in orexin neurons in the transgenic mouse brain, electrophysiological studies were performed using brain slice preparations from orexin/HaloR mice. Orange light illumination immediately hyperpolarized HaloR-expressing orexin neurons and completely inhibited the generation of action potentials. Firing was almost completely inhibited for approximately 10 s from the initiation of orange light illumination (Fig. 3). Inhibition was lasted for 1 min, however, firing gradually recovered suggesting that HaloR is not suitable to inhibit the activity of neurons for longer timescales. To obtain in vivo data, orexin neurons were acutely inhibited for 1 min in freely moving orexin/HaloR mice. To illuminate orange light into the hypothalamus, fiber optics were bilaterally inserted into the hypothalamus. To monitor sleep/wakefulness, EEG and EMG electrodes were implanted and recorded. Orange light was emitted by a strong light source such as laser or high-powered LED, and the light was guided through quartz fiber optics and connected to plastic fiber optics via an optical swivel (Fig. 4). Light illumination was applied when mice were in a state of arousal. Although mice maintained a wakefulness state for a few seconds even after the initiation of orange light illumination, EMG power gradually decreased and the slow-wave component of EEG increased. These facts indicate NREM sleep induction. The mice slept during orange light illumination, but they immediately returned to wakefulness when orange light was turned off. This result suggested that the acute inhibition of orexin neuronal activity is sufficient to promote the initiation of NREM sleep. However, phenotypes observed in narcolepsy, such as cataplexy and a direct transition from wakefulness to REM sleep were not observed in acute inhibition of orexin neurons for 1 min. Inhibition of orexin neurons for 1 min was not enough to reproduce all the phenotypes of narcolepsy. To further study the physiological significance of orexin neuronal activity in sleep/wakefulness regulation in conjunction with wake centers, EEG and EMG activity and the activity of dorsal raphe (DR) serotonergic neurons were recorded simultaneously. In vivo single unit extracellular recording was performed using conscious, head-fixed orexin/HaloR mice (Fig. 5). The firing of serotonergic neurons in the DR was recorded when orexin neuronal activity was inhibited by light illumination via fiber optics located in the hypothalamus. Serotonergic neurons in the DR are densely innervated by orexin neurons and are activated by orexin either directly or indirectly (Liu et al. 2002; Brown et al. 2002). During orange light illumination, the firing frequency of serotonergic neurons in the DR gradually decreased in conjunction with the EEG delta power increase. These observations suggest that acute silencing of orexin neuronal activity by illuminating orange light into the hypothalamus decreased the firing frequency of serotonergic neurons in the DR and induced NREM sleep in mice (Fig. 5). This study provided the first example that selective inhibition of orexin neurons is sufficient to induce a transition from wakefulness to NREM sleep (Tsunematsu et al. 2011).
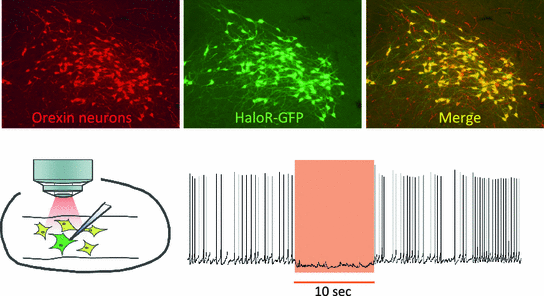

Fig. 3




Generation of transgenic mice in which orexin neurons express HaloR Immunohistochemical study confirmed that orexin-immunoreactive neurons (red) expressed HaloR-GFP (green). Slice patch clamp recording from orexin neurons confirmed the function of HaloR in orexin neurons. Orange light illumination from objective lens hyperpolarized membrane potential and inhibited generation of action potentials during illumination (Color figure online)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







