7 Oligodendrogliomas
Frequency and Incidence
According to data collected by the Central Brain Tumor Registry of the United States (CBTRUS 2007-2008), OD, OA, and anaplastic oligodendroglioma (AO) accounted for 5.1%, 2.8%, and 2.8% respectively of neuroepithelial tumors and 2.0%, 1.0%, and 1.1% of all primary brain tumors. Therefore low-grade oligodendroglial tumors are three times more frequent than anaplastic tumors, and in total represent 4.1% of tumors compared with 8.5% for astrocytomas.1 Assuming a population of just over 58 million people in the United Kingdom and an incidence rate of between 15/100,0002 and 21/100,0003 for all primary brain tumors, there are approximately 428 new cases of OD/OA/AO per year.
Histology
The realization that oligodendrogliomas are more chemosensitive than astrocytomas has increased the enthusiasm amongst neuropathologists to search hard for evidence of oligodendroglial differentiation in biopsy and resection specimens.4 This in turn has ignited considerable debate about the minimum criteria for OD/OA.
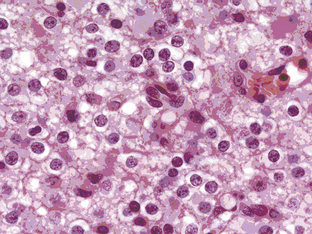
Oligodendrogliomas can present at any age and in any location in the brain, although they frequently occur in cortical locations in the frontotemporal lobes as partially calcified mass lesions. Macroscopically, they may be distinguished by the presence of a mucoid matrix that is softer than the firmer surrounding brain. They often infiltrate grey matter and change the appearance of the cerebral cortex. The most characteristic microscopic feature of OD are the sheets of uniformly rounded cells and perinuclear halos, giving the so-called fried egg appearance, due to a technical artifact whereby the cytoplasm swells up in paraffin sections after a delay in fixation (Figure 7-1). This is not always present, however, particularly if the tumor specimen is frozen prior to being embedded in paraffin, nor is this appearance pathognomic for OD, as it also occurs in ependymomas and central neurocytomas. The key pathological feature that distinguishes OD from astrocytomas is the fact that cell density and nuclear size and shape are much more uniform. Astrocytomas typically display considerable nuclear pleomorphism and, in particular, angulated nuclei with spindle-shaped cells. Another classic feature is the presence of a rich, thin-walled capillary network arranged in a way that resembles chicken wire. Other frequent features include calcification, either within the tumor or within the surrounding brain, and cortical invasion with perineuronal or perivascular cells, known as satellitosis.
Grade II oligodendrogliomas have a low mitotic index (less than 5%), although there may be well-circumscribed foci of higher cellularity, an occasional mitosis, and cellular atypia.5 By definition, OD cannot have “significant mitotic activity,” but this is a vague term and the exact number of mitoses has not been defined, although in a recent large series, a cutoff value of 6 to 10 mitoses per high power field correlated with reduced survival.6
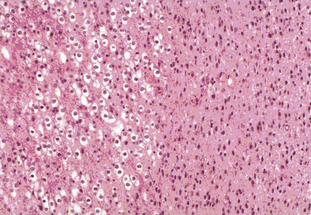
Tumors with a mixture of astrocytic and oligodendroglial components are called oligoastrocytomas (OA) or mixed gliomas, which, by definition, show divergent forms of glial differentiation. The two cell types may occur as regionally distinct and cytologically distinct populations of cells within the same tumor (Figure 7-2) or, more rarely, as intermixed populations containing cells with both astrocytic and oligodendroglial phenotypes. Many oligodendrogliomas contain GFAP-positive cells (i.e., cells of astrocytic lineage) and so the demonstration of astrocyte-like cells in an OD should not be regarded as necessarily diagnostic of an oligoastrocytoma, unless the proportion of the minority cell type exceeds some arbitrary figure ranging from 20% to 60%. Particular care should be taken when interpreting frozen specimens, as the process of freezing can change cells with an oligodendroglial morphology to cells with a more astrocytoma-like pattern with dark irregular nuclei. These tumors are regarded as morphologically ambiguous tumors and further characterization can only be carried out using molecular genetics. The variable criteria for the diagnosis of an OA has been succinctly reviewed by Burger.7
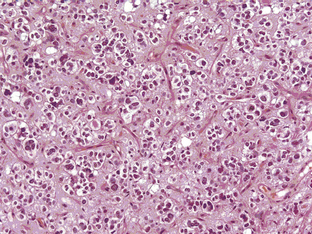
The grade III anaplastic OD (AO) still retains the characteristic nuclear uniformity of OD but is more cellular, and shows more nuclear atypia, frequent mitotic activity, and noticeable microvascular proliferation or hypertrophy (Figure 7-3). AO may present de novo or evolve from malignant transformation of a pure OD. Necrosis may be present and does not in itself indicate a grade IV glioma.8 The vessels are frequently thickened and hypertrophied but do not form the glomerular tufts seen in glioblastoma multiforme (GBM). Most AOs do not evolve into GBMs; however, there are occasional cases and, in these, it is not really possible to distinguish between a “highly malignant” AO and a GBM with some oligodendroglial features. In the most recent WHO classification (2007), anaplastic oligoastrocytoma (AOA) with necrosis was reclassified as GBM-O. Recent data from a large trial of AOs and AOAs suggest that these tumors have similar survival curves to that of GBM and confirm this reclassification. Whether this tumor is a separate clinicopathological and molecular genetic entity remains to be proven.
Oligodendrogliomas have a greater propensity for leptomeningeal spread than astrocytic tumors. This occurs in about 1% to 2% of cases, with systemic metastases also being occasionally reported. This may be a consequence of the longer survival of patients as compared to other glioma subtypes and of heterozygous deletions of CDKN2A/p 16 genes.9
Molecular genetics
Oligodendrogliomas are associated with specific genetic abnormalities, namely loss of the short arm of chromosome 1 (1 p) and the long arm of chromosome 19 (19 q). This was first demonstrated 10 years ago in a genomic wide analysis,10 and subsequently linked in retrospective studies to a durable response to a combination of procarbazine, CCNU, and Vincristine (PCV) as well as longer survival.11,12 In contrast, response rates for patients with isolated 1 p loss or no loss was less than 25%, although the presence of 1 p loss may also identify other treatment-sensitive malignant gliomas, including rare GBMs (see Table 7-1).13 The chromosomal locations involved in the favourable therapeutic response have been further narrowed down to lesions at 1p36 and 19q13.3.14 Of the two markers, 1 p has the greater specificity since 19 q losses have also been reported in high-grade astrocytomas and OA.15
TABLE 7-1 1 p and 19 q Losses versus Glioma Subtypes and Primary/Recurrent Status Adapted from Smith et al. 200013
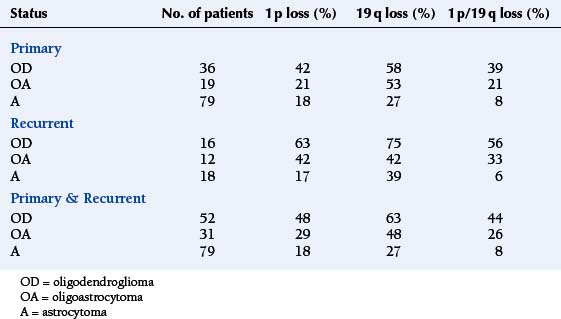
The frequency of these losses varies between different grades of glioma and different histological subtypes (see Figure 7-1). As can be seen, the 1 p/19 q loss is not specific to OD as it also occurs in OA and a minority of astrocytomas; however, it appears to be a statistically significant predictor of prolonged survival in patients with pure OD, irrespective of tumor grade, but not in patients with OA or astrocytomas.16 The presence of a typical perinuclear halo in more than 50% of tumor cells and a “chicken wire” vascular pattern is seen in more than 90% of tumors showing LOH on 1 p or 19 q.17
More recently, mutations of two enzymes involved in the Krebs cycle, isocitrate dehydrogenase (IDH) 1 and 2, have been described in 84% of OD, 94% of AO, and 100% of OA and AOA, making this an even more common molecular signature than 1p19q.18 These mutations are rarely seen in primary GBM.
Clinical Features and Natural History
Most ODs are slow-growing tumors; they usually present in adults between the ages of 30 and 50 with partial or generalized seizures. The incidence of seizures is lower in AO than in OD/OA.19 Occasionally, they occur in older patients. In these cases, they more commonly present with symptoms of an expanding, infiltrating mass lesion (raised intracranial pressure with or without focal neurological deficits).20 They are more likely to present as an intracerebral hemorrhage than other low-grade gliomas, probably because of their thin-walled capillary network.
ODs and other low-grade gliomas account for up to 15% of patients with medically intractable seizures.21 Seizure control may vary and, particularly in tumors occurring in the motor strip, be resistant to anticonvulsant polypharmacy.22 In this situation, patients may be considered for “epilepsy surgery” on symptomatic grounds rather than on pure oncological grounds. The relationship between the molecular genetics and their mode of presentation is not clear, but the clinical phenotype is presumably related in some way to the speed of growth and the duration of time that the tumor has been present in the brain. This is supported by the observation that there is an inverse relationship between tumor grade and frequency of seizures—as a general rule, the more benign the tumor, the more likely it is to be associated with intractable epilepsy.
The prognosis of OD is significantly better than for astrocytomas, with a median survival of 10 years or more. As with astrocytomas, there is a wide range of survival times, presumably due to underlying genetic factors. Like other low-grade tumors, favourable prognostic factors for OD include age less than 40 years, presentation with seizures, normal neurological examination and, in some reports, extent of resection.23,24 In a retrospective review of 106 patients with OD (n=77) and OA (n=29 ) from the Memorial Sloan Kettering Cancer Center in New York, the overall median time to progression was 5.0 years (0.5 –14.2) and the overall survival, 16.7 years.25 Neither of these two markers of outcome were significantly affected by treatment.
Imaging
Oligodendrogliomas and oligoastrocytomas are space-occupying lesions that have a predilection for frontal lobes and often infiltrate up to the cortical surface. There are no specific features that distinguish OD/OA from astrocytomas, although the presence of calcification on CT scans and, specifically, a gyriform pattern (due to calcification along the cortical ribbon) is highly suggestive. MR spectroscopy may, in time, be able to distinguish OD/OA from astrocytomas; a study of 15 patients with OD and AO showed that the level of glutamine plus glutamate was significantly higher than in low-grade astrocytomas.26 Similarly, a study of diffusion-weighted imaging (DWI) showed that ODs had significantly lower group apparent diffusion coefficient (ADC) values than astrocytomas (A) and that up to 83% of the subjects could be correctly classified into the OD and A groups by reference to an ADC histogram.27
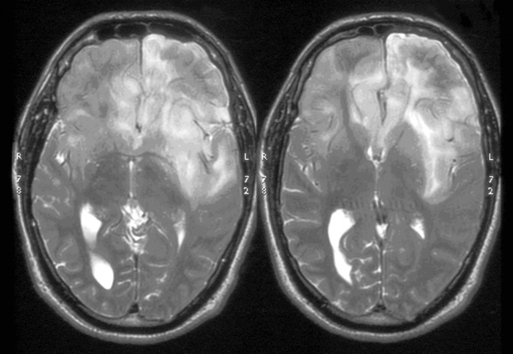
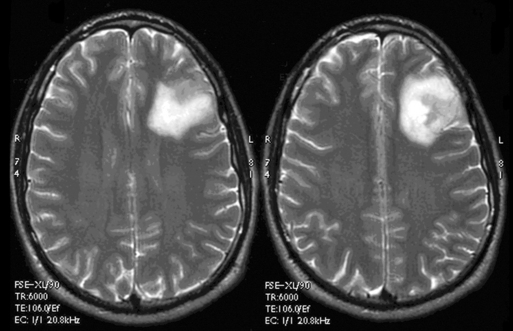
These tumors can be diffusely infiltrating (Figure 7-4) or well-demarcated (Figure 7-5), and appear on MR imaging as high-signal on T2W, PD, and FLAIR sequences and as intermediate-signal or low-signal on T1W sequences. Of these, FLAIR offers superior delineation of the tumor margins and is also better at showing different tumor components, at defining the borders of a postoperative cavity, and at demonstrating local spread to white matter tracts.28
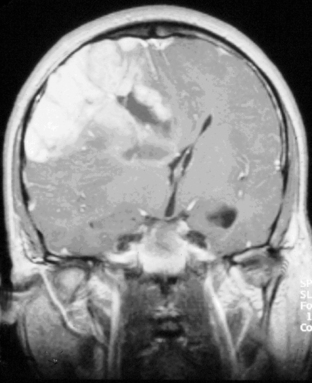
The presence of contrast enhancement in a glioma is often regarded as a sign of malignancy (Figure 7-6) and, in the case of OD, reduces the median survival from 11 years in nonenhancing tumors to 3 years in enhancing ones. For this reason, Daumas-Duport has suggested a grading classification for OD that includes the presence of contrast enhancement as a variable and which was found to be highly predictive of survival.29 Contrast enhancement reflects breakdown of the blood-brain barrier and correlates with angiogenesis. In a study examining the prognostic significance of both tumor enhancement and angiogenesis in OD, 10-year survival was 83% in patients with nonenhancing tumors, compared to only 14% in enhancing tumors; 79% of the tumors showing contrast enhancement had a high tumor angiogenesis index indicating a close relationship between contrast enhancement and endothelial surface area.30 Furthermore, a significant proportion of low-grade gliomas, and in particular OD, may display high relative cerebral blood volume (rCBV) foci on perfusion imaging not reflective of high-grade histopathology.31
Functional imaging, particularly using FDG-PET, has been adopted in some centers for the management of OD, in particular for determining the degree of malignancy and guiding the surgeon to the most malignant area, as well as for discrimination of recurrent tumor from radiation necrosis.32 However, whether it provides additional information over and above that of conventional MRI sequences is debatable.
The results of imaging are vital to determining the subsequent management of a patient with a radiologically suspected low-grade glioma. However, MRI scans may be falsely reassuring. Several studies have demonstrated that contrast enhancement in gliomas is only weakly predictive of tumor grade; in a study of 314 unselected patients with both malignant and low-grade gliomas, 58 lacked contrast enhancement and, of these, approximately one third were malignant, particularly in older patients.33 For this reason, biopsy is recommended for older patients presenting with seizures and a nonenhancing lesion, as they are more likely to be harbouring a high-grade glioma. For younger patients without neurological deficit whose tumor has not changed on a second scan 3 months after the first study, it is reasonable to adopt a watch and wait policy, although the precise scanning interval will vary according to local resources.
Imaging is therefore vital for monitoring the natural history of low-grade gliomas, particularly as these tumors are often not treated until there is clinical or radiological evidence of progression.34 Although it is well recognized that low-grade gliomas eventually undergo malignant transformation, very little is known about their radiological history and growth rates during their “stable” phase. In a study of 27 patients with untreated OD and OA, analysis of mean tumor diameters over time showed that these tumors were continuously growing with an average slope of 4.1 mm/year.35 We have extended this study to all types of grade II gliomas and have shown that even in nontransformers, an average growth rate of 13% per annum was observed, compared to a growth rate of transformers up until the penultimate scan (i.e., before transformation took place) of 26% per annum. This growth rate increased to 56% per annum in the final 6-month time period that included transformation, implying that low-grade gliomas that grow more rapidly in their “stable” phase are more likely to undergo malignant transformation.36