CHAPTER 195 Optic Pathway Hypothalamic Gliomas
Optic pathway hypothalamic gliomas (OPHGs) are included as one of the representative childhood supratentorial tumors. They represent 1% of all central nervous system (CNS) tumors and 3% to 5% of all pediatric brain tumors. OPHGs tend to affect infants and young children, who can have various clinical symptoms. Sixty-five percent of patients with OPHGs are younger than 5 years at diagnosis. They occur with equal frequency in males and females. OPHGs cause symptoms as a result of the anatomic structures involved, such as the optic pathway (including the optic nerve, chiasm, tract, and radiation), the hypothalamus-pituitary axis, the limbic system, the third ventricle, and the circle of Willis. When these structures are compromised by the glioma or by the treatments applied, children may suffer from visual, endocrine, cognitive, and psychobehavioral dysfunction. A mass in the third ventricle produces obstructive hydrocephalus, which may be the primary cause of symptoms. The majority of pediatric low-grade astrocytomas in the hypothalamic/chiasmatic region are typical pilocytic astrocytomas (PAs).1,2 There may be other gliomas such as fibrillary astrocytoma and ganglioglioma, but anaplastic astrocytoma and primitive neuroectodermal tumor are uncommon. PAs have a typical histologic pattern consisting of a biphasic pattern of Rosenthal’s fibers and eosinophilic granular bodies. These tumors are indolent in nature, and an aggressive course is rare. Grossly, they appear partly cystic and have a grayish homogeneous pattern with areas distinct from normal brain tissue. Cerebrospinal fluid (CSF) dissemination is uncommon, and malignant transformation is rare in PAs. A subgroup of PA, pilomyxoid astrocytoma (PMA), was described in 1999.3 PMA typically arises in the hypothalamus or optic chiasm of an infant or young child, with the mean age at initial evaluation being 10 to 18 months of age. PMA has histologic features that are quite unique and different from those of PA. The prominent histologic features of PMA are monotonous, bipolar spindle cells with an angiocentric arrangement within a strikingly myxoid background.3,4 PMA has more aggressive behavior than PA. A local recurrence rate after surgery of 55% to 76% has been reported.3–5 Dissemination through CSF has been demonstrated in 11% to 14% of patients with PMA. Chikai and colleagues speculated that PMA was an infantile form of PA. They suspected that a subset of PMA in the optic pathway/hypothalamus originates from the optic chiasm, possibly derived from radial glia existing in the embryonic optic chiasm.6
These tumors are often associated with neurofibromatosis type 1 (NF1). NF1 is predominantly autosomal dominant, but about 50% of cases result from new mutations. In patients with NF1, the frequency of OPHGs ranges from 6.6% to 20%,7–9 whereas in children with OPHGs, NF1 is diagnosed in about 27% to 58%.1,2 Recent guidelines by the Optic Pathway Glioma Task Force on Neurofibromatosis of the National Institutes of Health recommend a complete ophthalmologic examination at the diagnosis of NF1, with annual examinations until 6 years of age and longer interval thereafter because children younger than 6 years have the greatest risk for the development of symptomatic OPHG.10 However, more recent literature has revealed that OPHG in children with NF1 can be diagnosed after the age of 6 and may progress until the age of 12. These authors recommend more frequent observation than previously proposed.11,12
Clinical Findings
The initial signs and symptoms are dependent on the location and direction of tumor growth and the structures involved. The predominant clinical manifestations are visual disturbances, hydrocephalus, and endocrine dysfunction; the most common initial symptoms are visual complaints. Children may exhibit a decrease in visual acuity or, less often, visual field deficits. If the tumor is located in the intraorbital space, proptosis with deviation of the affected eye may occur. Suprasellar gliomas extending into the third ventricle often cause obstructive hydrocephalus. Of such gliomas, tumors of hypothalamic origin may not cause visual symptoms. Hydrocephalus is manifested as macrocephaly and failure to thrive in infancy. In older patients, the symptoms associated with hydrocephalus are headaches, emesis, lethargy, and decrease in school performance. Hypothalamic-pituitary axis involvement by the tumor leads to partial hypopituitarism, and about 50% of children have measurable endocrine abnormalities.2 Common endocrine dysfunctions are growth hormone deficiency and precocious puberty. Precocious puberty is more common in children with NF1 and OPHG and occurs in about 40% of cases.9 Diabetes insipidus is rare in children with suprasellar gliomas.
Age can play a factor in the type of initial signs seen. Infants often have macrocephaly, hydrocephalus, failure to thrive, or diencephalic syndrome. Younger children may have hydrocephalus, failure to thrive, visual disturbance, or precocious puberty. Older children can have hydrocephalus and visual disturbances as well. Diencephalon syndrome, originally described by Russel, is characterized by a suprasellar glioma in infancy that is manifested as lack of weight gain and emaciation with little subcutaneous fat tissue and alertness. These infants have normal oral intake and height and may exhibit nystagmus or head bobbing. Cappelli and coauthors reported that 6 of 69 children with optic pathway tumors had diencephalon syndrome,2 whereas 6 of 14 children with OPHG diagnosed when younger than 3 years exhibited diencephalon syndrome according to Silva and associates.13 CNS tumor is a rare cause of failure to thrive in childhood, but clinicians should keep it in mind when considering the differential diagnosis. The diagnosis of OPHG is often delayed, and these tumors can be quite large at discovery.14
Neuroimaging
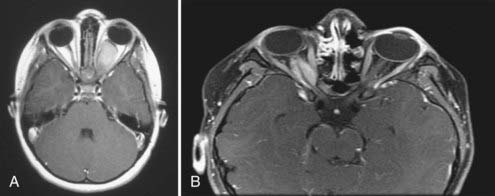
Although CT is useful, magnetic resonance imaging (MRI) is the imaging modality of choice for characterizing an OPHG. Benign gliomas are usually hypointense or isointense on T1-weighted images and hyperintense on T2-weighted images. These tumors generally have various degrees of enhancement ranging from none to strong enhancement with contrast agents. Cyst formation in or around the solid tumor may be present. Intraorbital optic nerve gliomas show fusiform enlargement of the nerve, as well as an elongated length, which results in a tortuous course and often kinking appearance (Fig. 195-1A). The rare optic nerve sheath meningioma in childhood may mimic optic nerve gliomas (Fig. 195-1B).
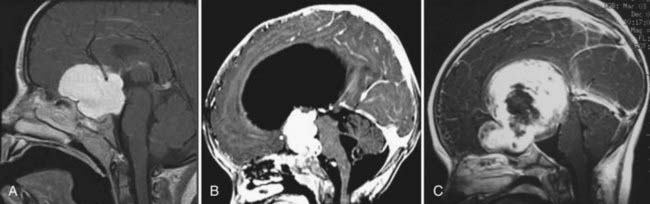
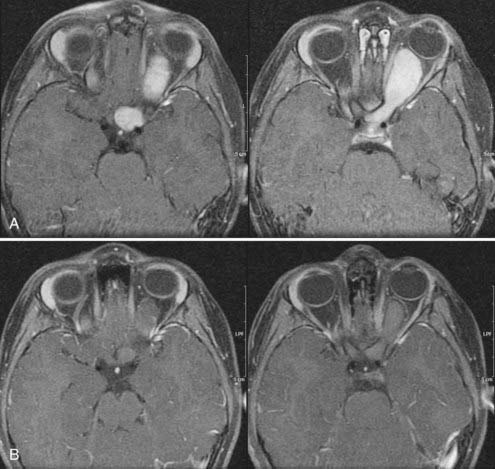
Small chiasmal tumors show expansion of the chiasm with little or no enhancement, which is often seen in children with NF1. Large chiasmal gliomas can exhibit exophytic extension into the suprasellar and parasellar locations and further dorsally into the third ventricle. MRI of these globular suprasellar gliomas shows that the chiasm and hypothalamus are often indistinguishable because of the lack of an anatomic border between these structures (Fig. 195-2A to C). These globular suprasellar gliomas often enhance after intravenous infusion of a contrast agent. They may be associated with cyst formation within or around solid tumor. Another distinct subgroup consists of a diffuse optic pathway glioma. It affects the entire optic pathway, including the optic nerve, chiasm, and tract and the lateral geniculate body. On rare occasion, the optic radiations are also involved, which is more common in children with NF1.15 A diffuse optic pathway glioma affects these structures bilaterally and is characterized by the “mustache” sign. In the children with NF1, it is common to find nonenhancing hyperintense lesions on T2-weighted images in the basal ganglia, brainstem, and cerebellum.16
Treatment
When the patient has symptoms or the tumor is progressing, or both, treatment is warranted. Although radical tumor resection is ideal, because of the anatomic structures present around the tumor, aggressive surgery would compromise visual, neurological, and endocrine function, and total resection is therefore not possible without complications. In one study involving surgical resection of optic pathway gliomas, the progression-free survival rate at 5 years was 52.4%,17 so approximately half of the patients had no recurrence for 5 years.
Surgical Treatment
Optic Nerve Glioma
In the past, one of the indications for surgery on optic nerve gliomas was to prevent posterior extension to the chiasm and beyond. However, orbital optic nerve gliomas rarely extend posteriorly after diagnosis.18 Only 5% of optic nerve gliomas recur in the chiasm after “complete” intraorbital excision.19 The present therapeutic recommendation for symptomatic or progressive optic nerve glioma, or both, is chemotherapy (Fig. 195-3A and B). Children with no useful vision, severe eye-threatening proptosis, or a painful eye that failed treatment with chemotherapy may benefit from tumor resection. If both eyes are affected, no indication for surgery is present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree