Chapter 88 Ossification of the Posterior Longitudinal Ligament
Ossification of the posterior longitudinal ligament (OPLL), most typically found in males (2:1) in their mid 50s, contributes to approximately 25% of cervical myelopathy in the North American population and higher percentages in the Asian population.1,2 Originating as early hypertrophy of the PPL with accompanying punctate ossification centers (early OPLL), these foci coalesce and become loci of frank ossification in the PPL.1,2 Early OPLL appears in patients in their mid 40s, whereas classic, more ossified OPLL is found in people in their mid 50s and later. OPLL may be managed with either anterior surgery (single/multilevel corpectomies with fusion, with or without combined posterior instrumentation), or posterior surgery (laminectomy with or without fusion or laminoplasty) (Figs. 88-1 to 88-6).
Prevalence of Ossification of the Posterior Longitudinal Ligament
In asymptomatic North Americans, the frequency of OPLL is reportedly 0.12% on plain radiographs, and in the Japanese, it is 2.2%. In Koreans, 0.6% of 11,774 adults demonstrated OPLL involving the C3, C4, and C5 on plain radiographs; 32% had continuous OPLL, 31% segmental OPLL, 31% mixed OPLL, and 5.6% focal OPLL.3 In the presence of myelopathy, OPLL is evident, using CT and MRI studies in up to 25% of North Americans and in at least 27% of Japanese.4–6 Seventy percent of cervical OPLLs involve 2.7 to 4 levels, progressing in a caudal-rostral fashion, and the remaining 30% are evenly divided between the proximal thoracic (T1-4) and the proximal lumbar (L1-3) regions.
Genetics of Ossification of the Posterior Longitudinal Ligament
Human Leukocyte Antigen: Probable Site on Genome
Multiple studies increasingly document a genetic correlation among OPLL, diffuse idiopathic skeletal hyperostosis (DISH), and ossification of other ligaments—ossification of the yellow ligament (OYL) and ossification of the anterior longitudinal ligament (OALL).7,8 In evaluation of 91 sibling pairs of patients with OPLL in 53 Japanese families, the genetic locus for OPLL was found near the human leukocyte antigen (HLA) site on chromosome 6-p. In patients with DISH, 50% have concurrent OPLL and test positive for HLA.9 In the Chinese population, another susceptibility gene, COL6A1, appears to represent a “common susceptibility gene” for both OYL and OPLL.10
Autosomal Dominant Inheritance
An autosomal dominant mode of inheritance is supported by genetic and epidemiologic data from patients with OPLL. OPLL is found in 26.15% of parents and 28.89% of siblings of patients with OPLL, a finding that supports a likely autosomal dominant transmission.11 A 53% expression rate of OPLL is observed in patients with two concurrent HLA strands compared with 24% with one strand, which further supports this hypothesis.8 Autosomal recessive transmission has been suggested when 56% of a patient’s siblings with both HLA haplotypes were symptomatic with OPLL, whereas those with only one HLA haplotype were not.12 Two additional unique genetic factors were identified in 18 patients with OPLL compared with 51 age-matched controls: BamHI 10.0/10.0 kb and HindIII 19.0/19.0 kb genotypes.7,13
Other Contributing Factors (Hormones and Proteins)
Genetically modulated hormones and proteins also appear to contribute to the expression of OPLL. Increased concentrations of growth hormone receptors and activins have been shown to enhance OPLL expression/progression.14,15 Elevated concentrations of bone morphogenetic proteins have resulted in increased osteogenesis in originally nonossified ligaments of OPLL patients (347 families, 1030 relatives).15 In a more recent study, bone morphogenetic protein 2 (BMP-2) additionally positively correlated with the extent of OPLL progression.16 Insulin was also correlated with OPLL onset/progression in people with non–type 1 diabetes via direct and indirect stimulation of BMP-2 within the ligament.17 Fibronectin, a glycoprotein that plays a role in the development of bony tissues, was also found to be significantly elevated in patients with OPLL or OYL.18
Anatomy of Ossification of the Posterior Longitudinal Ligament
In Vitro Characteristics of Cultured Posterior Longitudinal Ligament in Patients with Ossification of the Posterior Longitudinal Ligament
In patients with OPLL, the PPL is osteogenic.7,8,13–15 Immunohistochemical evaluation of PPL cells for patients undergoing anterior cervical decompression for cervical disease revealed “up-regulation of proliferating cell nuclear antigen” in patients with OPLL.19 This finding of increased osteogenicity of the PPL was also confirmed in myelopathic patients with OPLL in North America.20 Collecting supernatants of PPL obtained intraoperatively from patients with OPLL compared with non-OPLL control patients (with spondylosis) revealed increased osteocalcin synthesis in the OPLL patients. The quantity of osteocalcin induced was determined by incubating these PPL cells with 1.25(OH)2 and vitamin D3 for 72 hours in serum-free medium.21 Those with OPLL grew to confluence, whereas those with spondylosis alone did not respond to vitamin D3 priming.
Classification of Ossification of the Posterior Longitudinal Ligament
Early Disease
OPLL represents a continuum of maturation that starts with hypertrophy of the PPL and ends with frank ossification.1,4,6,22 Early OPLL usually originates opposite multiple interspaces in patients in their mid 40s and is often misdiagnosed as multiple disc herniations. However, unlike disc herniations, OPLL begins with retrovertebral extension that can be seen on enhanced MRI studies performed with gadolinium (Gd)-DTPA and as punctate CT-documented ossification. Too often, patients undergo multilevel anterior discectomy and fusion procedures where the PPL is ignored, leading to many retained disc fragments, and in early OPLL, leaving patients with continued symptomatology.
Classic Disease
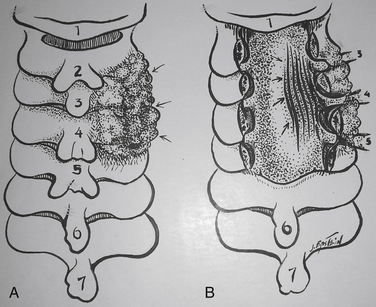
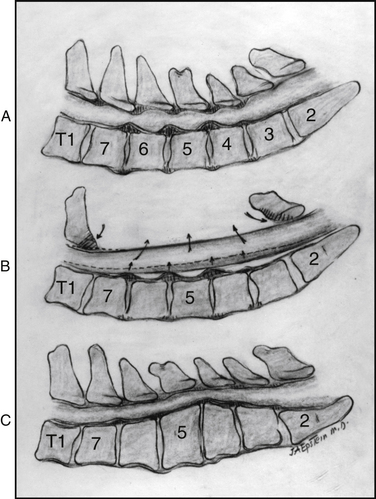
There are four classic types of mature/classic OPLL23 (Figs. 88-7 and 88-8). The segmental variant (39%), located behind the vertebral bodies, does not cross the intervening disc spaces. The continuous type (27%) extends from vertebra to vertebra, traversing the disc spaces. The mixed form (29%) simultaneously includes both continuous and segmental elements with “skip” areas. The “other” form (5%) is localized to the disc spaces, with limited degrees of rostral and caudal retrovertebral extension.
Myelopathy Scales
Japanese Orthopaedic Association Scale
The JOA scale categorizes the severity of myelopathy using a 17-point scale.24–27 In 2007, the JOA released a new evaluation tool for cervical myelopathy called the Cervical Myelopathy Evaluation Questionnaire (JOA CMEQ).24–27 The new tool consists of five categories, which include 24 questions devoted to upper extremity motor function, lower extremity motor function, bladder function, cervical spine function, and quality of life. The English version of JOA CMEQ, its calculation software, and users’ manual are available at the JOA home page (http://www.joa.or.jp/english/english_frame.html).
Clinical Presentation of Ossification of the Posterior Longitudinal Ligament
Patients with early OPLL become symptomatic in their mid 40s with mild radiculopathy/myelopathy, whereas those with classic (mature) OPLL are more typically affected in their mid 50s, presenting with more advanced myelopathic syndromes. Males are affected twice as frequently as females. Although most patients become subacutely symptomatic over a progressive 12-month period, 10% present with acute deterioration associated with minor trauma.28 In a series of 118 OPLL patients, minor trauma resulted in new myelopathy (13 patients), worsening of preexisting myelopathy (7 patients), or no new changes (7 patients). Eighteen of 19 patients with the narrowest cervical canals were most adversely affected.28 In another series of 91 patients operated on for OPLL, 26 sustaining minor trauma preoperatively experienced major myelopathic deficits postoperatively.29 Patients with more mobile spines associated with segmental, mixed, and other forms of OPLL exhibited poorer outcomes when compared with those with more rigid spines attributed to continuous OPLL. Typical symptoms included neck/arm pain or dysesthesias (>50% of the patients), and neurologic signs included arm/leg weakness (25%), spasticity, and ataxia.
Neurodiagnostic Studies in Ossification of the Posterior Longitudinal Ligament
Radiography
Based on lateral 6-foot plain radiographs, the normal anteroposterior dimension of the cervical spinal canal should measure 17 mm between the C3-7 levels. In absolute stenosis, the canal is narrowed to 10 mm or less, whereas with relative stenosis, the canal measures between 10 and 13 mm. The extent of OPLL is readily described by the occupancy ratio, which is determined by dividing the thickness of the measured ossified lesion by the anterior/posterior developmental canal diameter. If the ratio is greater than 40%, the risk of myelopathy increases.2
Computer-assisted measurements based on plain radiographs may be used to follow the postoperative progression of OPLL.30 In a multicenter study, lateral radiographs taken immediately as well as 1 and 2 years following posterior decompressions were assessed (131 patients). All radiographs were transformed into digital images and compared. Over 2 years, there was a 56.5% rate of progression, occurring more often in younger patients with the mixed and continuous forms of OPLL.
Magnetic Resonance Imaging
MRI examinations, particularly T1- and T2-weighted MRI studies, performed with and without contrast (Gd-DTPA) demonstrate the spinal column, spinal cord, nerve roots, intrinsic cord disease, and extrinsic cord compression from the occiput through the cervicothoracic junction in the transaxial, coronal, and sagittal planes. On noncontrast MRI studies, hypertrophied PPL often appears opposite multiple disc spaces, demonstrated by accompanying retrovertebral extension appearing slightly hyperintense and enhancing with Gd-DTPA. Classic OPLL is identified on 50% of T1-weighted MRI studies by a hyperintense signal, reflecting the presence of fat within mature Haversian canals actively engaged in bone marrow production. MRI examinations may also help identify disc herniations (hypointense masses) that occur in 81% of cases in conjunction with segmental OPLL.31
Ossified OPLL, which appears hypointense on MRI studies, may lead to an underestimation of the true extent of the OPLL and should therefore be combined with plain radiographic and CT-based studies to document the true degree of cord compression more accurately. In a multicenter study, 156 OPLL patients from 16 institutions were followed an average of 10.3 years with plain radiographs, MRI, and CT studies.32 Of interest, all 39 patients with OPLL occupying greater than 60% of the anteroposterior diameter of the spinal canal were myelopathic, whereas only 49% with OPLL occupying less than 60% of the spinal canal were myelopathic.
Intrinsic cord swelling/edema, myelomalacia, and gliosis produce hyperintense signals on T2-weighted MRI scans and are considered poorer prognostic signs for cervical spondylotic myelopathy (CSM) compared with OPLL.31,33 Despite the 43% incidence of increased preoperative cord signals on MRI studies that failed to resolve postoperatively, patients with OPLL exhibited better outcomes compared with those with CSM. Dynamic MRI studies may also prove useful in demonstrating “dynamic” compression preoperatively and residual cord compromise postoperatively.
Computed Tomography
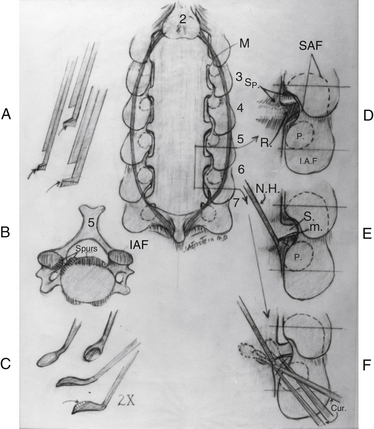
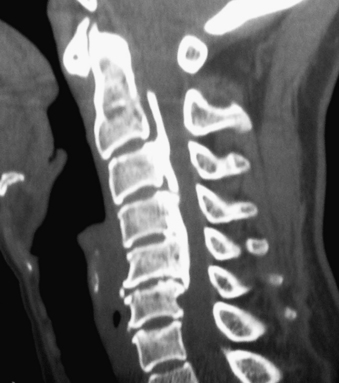
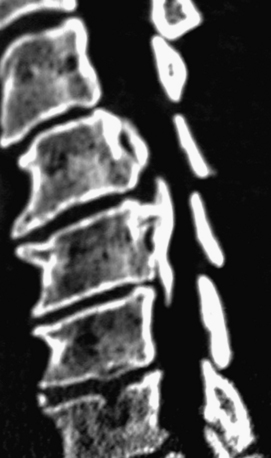
Noncontrast CT, intravenous-enhanced contrast CT, two-dimensional and three-dimensional reconstructed CT, and myelo-CT studies directly demonstrate punctate ossification characteristic of early OPLL or frank ossification typical of classic OPLL (Fig 88-9; see also Figs. 88-7 and 88-8). CT studies also demonstrate degenerative changes adjacent to levels of prior surgery.33 (Double-dose intravenous contrast-enhanced CT images may increase resolution of lateral or foraminal root pathology, helping to differentiate postoperative scar [enhancing] from new disc pathology [nonenhancing]). Two-dimensional and three-dimensional noncontrast CT reconstructed images provide a sagittal overview of the extent of cord compression without incurring the risk associated with myelo-CT studies. In particular, younger patients with OPLL and normal-sized spinal cords are at greater risk for acute deterioration following myelography compared with older individuals with OPLL and significant underlying cord atrophy.
Signs of Dural Penetrance on Computed Tomography
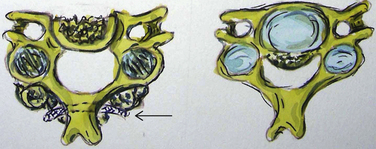
Bone-window CT examinations document two major signs of dural penetrance; the double-layer and single-layer CT signs (see Figs. 88-7 to 88-9). The double-layer sign is characterized by a hyperdense line of OPLL directly behind the vertebra, followed by a hypodense mass representing penetrated dura, and finally, an intradural hyperdense mass of OPLL.34,35 The single-layer sign is represented by a large central mass of OPLL. However, when the single mass is lateralized, the resulting positive C sign reflects an imbrication of the lateral dura and a greater likelihood for cerebrospinal fluid (CSF) fistula formation.
The double-layer sign is most highly correlated with absent dura, with CSF fistulas occurring between 52.6% and 84% of the time, whereas the single-layer sign (C sign) produces CSF fistulas 13.6% to 25% of the time. In one series, 10 of 12 OPLL patients with CT-documented double-layer signs developed dural defects at surgery, whereas only 1 of 9 patients showing the single-layer sign developed a CSF leak intraoperatively.35,36 As anticipated, the greatest focus of OPLL compression in these patients corresponded to the site of dural penetrance. From a series of 197 Korean patients undergoing anterior resection of OPLL, signs of dural penetration were observed in 30.5% of patients: 52.6% with double-layer signs (nonsegmental OPLL) and 13.6% with single-layer signs.36 For those with double-layer signs, the thicker the central mass of OPLL, the greater the incidence of intraoperative CSF fistula.
The majority of patients with OPLL who exhibit neither the single- nor the double-layer signs should not develop intraoperative CSF fistulas if careful dissection is carried out under an operating microscope. When Epstein performed multilevel simultaneous anterior corpectomies with posterior fusions in 54 OPLL patients, only one demonstrated the double-layer CT sign and one demonstrated the single-layer sign.34,37 In 85 similar patients, three CSF fistulas resulted, with one double-layer CT sign and two single-layer CT signs.34 In a more recent series of 110 patients undergoing similar procedures, five CSF fistulas resulted, with three with double-layer CT signs and two with single-layer CT signs.
Myelo-Computed Tomography Studies
Myelo-CT studies are now rarely performed, because combined MRI/CT studies are extremely accurate and avoid myelography’s inherent risks of precipitating neurologic deterioration. Dorsally and dorsolaterally, myelo-CT studies (dynamic, static) demonstrate shingling of the lamina, OYL, OPLL, spondylosis, and disc pathology contributing to significant cord compression/stenosis. Postoperatively, myelo-CT studies also confirm whether the posterior decompression has been adequate or if further possible anterior surgery is warranted. Following open-door laminoplasty performed in OPLL patients, there was less dorsal shift of the cord but greater cord expansion, the latter factor positively correlating with outcome.38
Studies Documenting Fusion Following Multilevel Anterior Cervical Surgery
Fusion criteria, documented on static and two-dimensional CT studies performed immediately postoperatively and repeated at 3, 6, and 12 months postoperatively included (1) the presence of bony trabeculation, (2) lack of bony lucency at the fibula strut allograft/vertebral body interface, and (3) ingrowth of bone centrally into the fibula. Dynamic radiographs additionally revealed (1) less than 3.5 mm of translation, (2) less than 5 degrees of angulation, and (3) less than 1 mm of motion demonstrated between the tips of adjacent spinous processes. An additional criterion for fusion following multilevel ACF performed with fibula strut allograft consists of cephalad or caudad bony ingrowth from the vertebral end plate into the central canal.39 Eighteen patients with OPLL had, on average, a 2.9-level ACF performed with fresh-frozen fibula strut allografts, accompanied by C2-T1 posterior wiring and fusion (PWF). CT documentation of bony ingrowth and other signs of fusion were observed in 17 (94%) of 18 patients (ingrowth documented utilizing 500–900 Hounsfield units). Bony ingrowth doubled from 3 to 6 months postoperatively, increasing both rostrally (1.5–3.5 mm) and caudally (2.1–4.6 mm). Although early bony ingrowth signaled progression toward fusion, its absence was not pathognomonic for a failure to fuse as observed in the one patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree