CHAPTER 60 OTHER SECONDARY HEADACHE DISORDERS
Although most patients who present to a physician with the chief complaint of headache have primary headache disorders, the clinician must always consider the possibility of a secondary headache. Thus, when evaluating a headache patient, the clinician must search for features that may serve as evidence for an underlying disorder. Such evidence may be found during the patient’s interview and physical examination. Although a list of these “red flags” is potentially exhaustive, common worrisome features include new-onset persistent or progressive headache, change in the characteristics of prior headaches, progressive nature, older age of the patient, worsening or precipitation by changes in posture or by the Valsalva maneuver, associated systemic or neurological symptoms, sudden and severe onset, and a history of trauma (Table 60-1). Abnormalities found on examination, including alterations in blood pressure, fever, neurological deficits, papilledema, and meningismus, may also raise this suspicion.
HEADACHE SECONDARY TO CEREBROVASCULAR DISORDERS
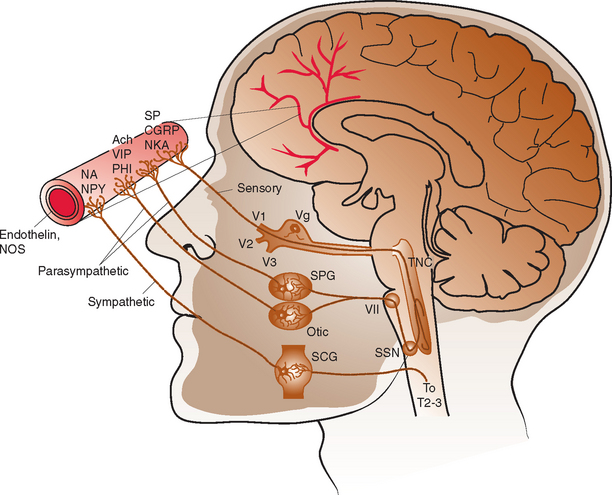
The intracranial vasculature is innervated by the sympathetic, parasympathetic, and sensory nervous systems (Fig. 60-1). The sensory system is the major conduit by which head pain is perceived after vascular stimulation. The majority of sensory nerves that innervate the anterior portion of the intracranial circulation terminate in the trigeminal nucleus caudalis, projecting via the first division of the trigeminal nerve, whereas those that innervate the posterior circulation terminate in the superior cervical ganglia and dorsal vagal ganglia.1–3 The density of sensory afferent vessels is greater in the posterior than anterior circulation, and thus stimulation of the posterior circulation is more likely to result in head pain.4–6
Direct stimulation of the intracranial vasculature and sinuses has been shown to cause activation of the trigeminal system. Stimulation of the superior sagittal sinus results in increased activity in the trigeminal nucleus caudalis, upper cervical dorsal horn, and thalamus.7 In addition, activation of orofacial excitatory receptive fields, mostly within the distribution of the first division of the trigeminal nerve, results in activation of thalamic neurons.8 The same thalamic neurons are also activated by stimulation of the superior sagittal sinus and/or the middle meningeal artery. Such evidence, in conjunction with a large body of additional data, characterizes the convergence and overlap within the trigeminovascular system. This continuity explains how nociceptive input at any part of the trigeminovascular system, including the intracranial vasculature, may result in pain in the head, face, and neck.
Ischemic Stroke
Approximately 25% of patients with stroke develop associated headaches, at least one half of which occur before the onset of neurological deficits.9 However, such headaches commonly go unrecognized, being overshadowed by more worrisome focal neurological symptoms. Headaches associated with ischemic stroke can be quite varying in their characteristics. Patients who have a primary headache disorder may develop a headache that closely resembles their usual headache. In those without such a history, headaches are most often pressing or throbbing in quality. The location of the headache may correlate with the site of the stroke. When headaches are unilateral, they are generally ipsilateral to the side of the stroke.4 Frontal pain is more common with anterior circulation strokes, and occipital pain is more common with posterior circulation strokes.
Headaches associated with stroke occur more frequently in young women and in persons with a history of migraine.10 Headaches are more common with large ischemic strokes and with strokes located in the territory of the posterior circulation, particularly those in the cerebellum.5,10 Headaches are much less common with subcortical infarctions, lacunar infarctions, and transient ischemic attacks.5,9
Cervical Artery Dissection
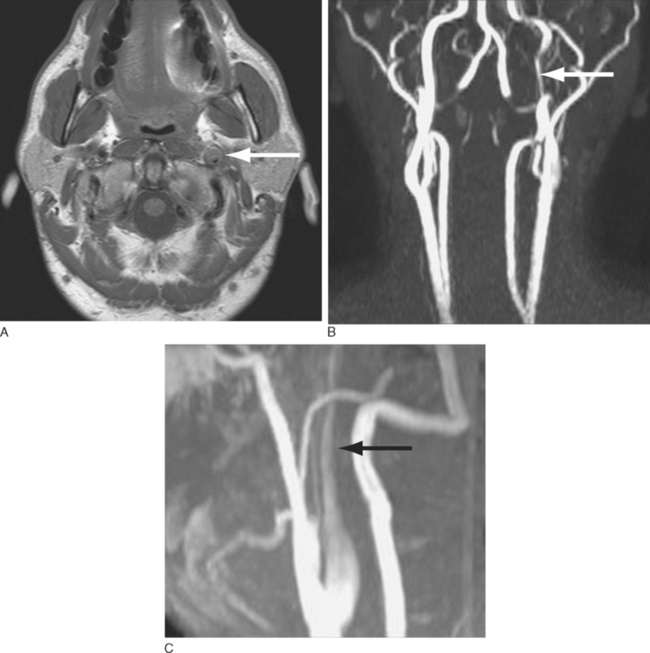
Headache is the most frequent presenting symptom of cervical artery dissection. Headache occurs with 60% to 95% of carotid artery dissections and with 70% of vertebral artery dissections.11 These headaches usually have a slow and gradual onset. However, about 20% of patients have a sudden and severe onset of pain consistent with that of a thunderclap headache.12 Headaches are typically located ipsilateral to the dissected artery. International Headache Society (IHS) diagnostic criteria stipulate that headaches considered secondary to cervical artery dissection must be ipsilateral to the dissection.13 Headaches associated with carotid dissection tend to be located in the ipsilateral frontotemporal region, lower face, jaw, or ear, whereas those of vertebral dissection are more often located in the parieto-occipital region. Patients with cervical artery dissection may also have neck pain. Neck pain occurs in 50% of patients with vertebral artery dissections and in 25% of patients with carotid artery dissections. Headaches associated with cervical artery dissections tend to be short-lived, with a median duration of 3 days. When longer lasting, headaches associated with carotid dissection usually resolve within 1 week, and those associated with vertebral dissection, by 5 weeks. Only an occasional patient develops persistent headaches after arterial dissection.
The diagnosis of cervical artery dissection may be accomplished by ultrasonography, magnetic resonance imaging (MRI), magnetic resonance angiography, computed tomographic angiography, and/or conventional catheter angiography (Fig. 60-2). MRI with diffusion sequences allows for detection of cerebral infarction that may occur secondarily to decreased perfusion or emboli distal to the dissection site. The treatment of cervical artery dissection varies according to the clinical presentation and location of disease, but it may include clinical observation, anticoagulation, and/or surgical intervention, including the use of arterial stents. Asymptomatic cervical artery dissections generally do not necessitate any intervention, although antiplatelet therapy would be recommended by some authorities. Symptomatic dissections may be treated with antiplatelet therapy or anticoagulation to prevent thrombus formation and the potential for subsequent artery-to-artery embolism.14,15 Although some studies suggest that anticoagulation may be associated with a lower rate of recurrent transient ischemic attacks and stroke, there is no controlled evidence to support the use of any particular antiplatelet or antithrombotic treatment in these patients.16,17 Anticoagulation is associated with an increased risk of hemorrhage that in select situations, such as intradural extension of the dissection, may be a contraindication to its use. Patients with aneurysmal dilation, SAH, significant arterial stenosis, or progressive neurological sequelae despite medical management may require surgical intervention.18
Intracranial Aneurysms
Headaches are common in patients with cerebral aneurysms. Headache is the presenting symptom of an unruptured intracranial aneurysm in 20% to 33% of patients.19,20 With the exception of a sentinel headache of the thunderclap type, no specific headache characteristics facilitate discovery of an unruptured aneurysm. Headaches may be focal or diffuse, unilateral or bilateral, anterior or posterior, and acute or gradual in onset.21 Therefore, it is imperative to check for associated neurological signs, such as cranial nerve palsies, that may provide evidence for an underlying lesion.
The IHS diagnostic criteria for headaches secondary to saccular aneurysms stipulate that the headache must be acute and of new onset and must resolve within 72 hours (Table 60-2).13 It is necessary to rule out SAH and intracerebral hemorrhage as underlying etiologies. Thunderclap headaches occurring in patients with an unruptured intracranial aneurysm must be recognized as warnings of impending aneurysm rupture. This type of headache is discussed in more detail later in this chapter.
TABLE 60-2 Headache Secondary to Intracranial Aneurysm: International Headache Society Diagnostic Criteria
A. Any new acute headache, including thunderclap headache and/or painful third nerve palsy fulfilling criteria C and D |
Subarachnoid Hemorrhage
Headache is common in patients with aneurysm rupture and occurs in association with nausea and vomiting in 75% of patients.22 SAH headaches are most often of the thunderclap type, sudden and severe at onset. Although there are numerous causes of thunderclap headaches, every patient who presents with this type of headache should be evaluated for SAH (Table 60-3, Fig. 60-3). Although there may be a focal pain distribution when the headache of SAH begins, the pain usually generalizes and becomes bilateral. Severe pain is usually short-lived, lasting 1 to 2 hours, followed by a less severe headache of longer duration. With small hemorrhages, headaches tend to resolve after 2 to 3 days, whereas those associated with larger hemorrhages last an average of 8 days. Neck stiffness and pain, elevated body temperature, and alterations of consciousness are common associated features. Additional neurological symptoms and signs include nausea and vomiting, focal motor deficits, seizures, coma, cranial nerve palsies, papilledema, ocular hemorrhages, visual field deficits, and paresthesias.
TABLE 60-3 Thunderclap Headache: Causes
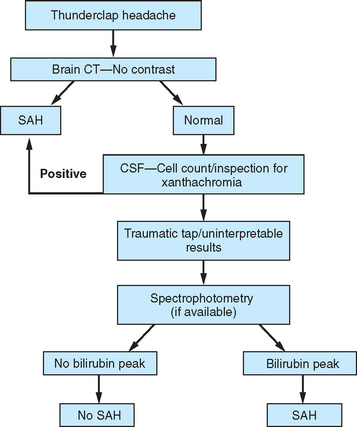
Figure 60-3 Evaluation for subarachnoid hemorrhage (SAH). CSF, cerebrospinal fluid; CT, computed tomography.
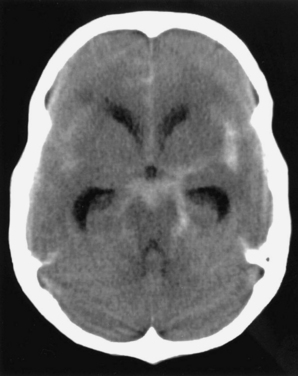
A history of a sentinel or warning headache is reported by 10% to 43% of patients with aneurysmal SAH.23 A sentinel headache is similar to the headache of SAH, but it occurs days to weeks before aneurysm rupture. Sentinel headaches generally develop over a few seconds and reach maximal intensity within minutes. Common associated features of SAH, including stiff neck, focal neurological symptoms and signs, and alterations in consciousness, are usually absent. Sentinel headaches may be caused by small aneurysmal leakages or stretching of the aneurysm wall without seepage of blood into the subarachnoid space. Identifying a sentinel headache as a warning of future SAH may allow for identification of an unruptured aneurysm and the need for surgical or endovascular intervention, thus avoiding a potentially catastrophic hemorrhage. Unfortunately, sentinel headaches are often ignored by patients and physicians, or they are misdiagnosed. According to a review by Edlow and Caplan, up to 50% of patients with SAH are initially misdiagnosed.24 Misdiagnosis occurs because the diagnostician fails to recognize the full clinical spectrum of SAH, lacks knowledge regarding the sensitivity of brain computed tomography (CT), and either fails to perform lumbar puncture or misinterprets CSF results.
CT of the brain is the first test in the evaluation of SAH (Fig. 60-4). CT is most sensitive early in the course of SAH. Its sensitivity is near 100% within the first 12 hours, but it decreases to approximately 50% by 1 week later.24–28 If CT yields negative results for SAH and does not provide an alternative diagnosis, lumbar puncture must be performed. Lumbar puncture evaluation should include measurement of opening pressure, cell count, visual inspection for xanthochromia, and spectrophotometry when available. When lumbar puncture is performed at least 12 hours after the onset of hemorrhage, the sensitivity of spectrophotometry is greater than 95%.29
Cerebral Venous Sinus Thrombosis
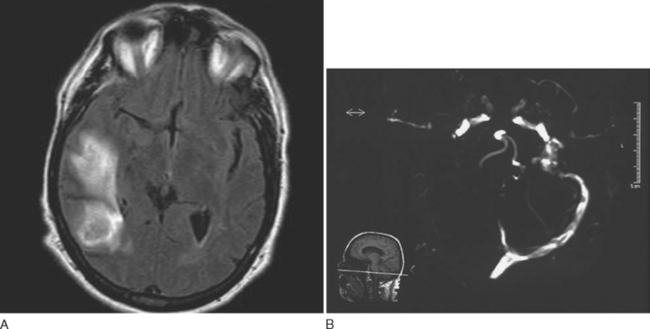
Headache is the most common presenting symptom of CVST, occurring in 75% to 95% of patients.30,31 In addition to headaches, patients with CVST usually present with papilledema, seizures, bilateral focal deficits, and/or altered level of consciousness. However, about 15% of patients present with isolated headaches.30,32 The headaches of CVST may be localized or diffuse, persistent, exacerbated by the Valsalva maneuver, and positional, with worsening on recumbency. Headaches of CVST have a gradual, subacute onset. However, approximately 10% of patients present with a headache of severe and sudden onset.33
Approximately 25% of patients with CVST who have normal neurological examination findings also have a normal CT, but CT is normal in fewer than 10% of patients who have focal neurological signs.34,35 Lumbar puncture should be performed, and opening pressure should be measured. Although 30% to 50% of patients with CVST have a combination of lymphocytic pleocytosis, elevated red blood cell count, and elevated protein levels, approximately 40% exhibit only an elevated opening pressure.36,37 MRI with venography or conventional angiography are the diagnostic modalities of choice when CVST is suspected (Fig. 60-5).
Anticoagulation is the treatment of choice for patients with CVST. Although anticoagulation carries the risk of promoting hemorrhage, it may prevent venous infarction, neurological worsening, and pulmonary embolism. Treatment with anticoagulation has been shown to be safe and to result in reduction in the risk of death or morbidity.38 If patients deteriorate despite adequate anticoagulation, thrombolysis via local infusion of thrombolytics into the occluded sinus and/or mechanical disruption of the thrombus should be considered.39
Giant Cell Arteritis
Headache is the most common manifestation. It is the presenting symptom in 48% of patients with GCA and occurs at some point during the disease course in 90%.40 The headache of GCA may be quite variable in its quality and location and may closely resemble a primary headache disorder. Associated symptoms, in descending order of frequency, are listed in Table 60-4.41,42 Ophthalmological complications include amaurosis fugax, visual loss, diplopia, ptosis, visual hallucinations, orbital bruits, and acute ocular hypotony.43 Visual loss tends to occur early in the course of the disease. If one eye is affected, the second is often affected within 2 weeks but rarely after 2 months.43
TABLE 60-4 Giant Cell Arteritis: Associated Clinical Features
Inflammatory markers, such as erythrocyte sedimentation rate (ESR) and C-reactive protein level, are measured when GCA is suspected. Most patients with GCA have significant elevations in these inflammatory markers. The odds of a positive result of a temporal artery biopsy are increased 2.0 times when the ESR is 47 to 107 mm/hour, 2.7 times when the ESR is greater than 107 mm/hour, and 3.2 times when the C-reactive protein level is above 2.45 mg/dL.44 C-reactive protein level is more sensitive than ESR for the detection of GCA, and a combination of the two tests has the greatest specificity.44 Depending on the results of these laboratory tests and clinical suspicion for the disorder, temporal artery biopsy, the diagnostic “gold standard,” may be required. Although biopsy may be performed unilaterally or bilaterally, it is essential that the specimen be of adequate length in order to maximize test sensitivity. Treatment with steroids should not be delayed while biopsy is awaited. When the suspicion for GCA is high enough to plan a temporal artery biopsy, prednisone should be started immediately to decrease the risk of vascular complications, including visual loss. Furthermore, the yield of temporal artery biopsy is not significantly decreased when performed within 1 to 2 weeks of steroid initiation.45 Although temporal artery biopsy is considered the “gold standard” diagnostic test for GCA, as many as 15% of patients who meet clinical criteria for GCA have negative findings on temporal artery biopsies.46 Such patients are less likely to have jaw claudication, an abnormal temporal artery on physical examination, constitutional symptoms, and significant elevations in inflammatory markers.46,47
Primary Angiitis of the Central Nervous System and Reversible Cerebral Vasoconstriction Syndrome
PACNS is a vasculitis that is limited to the central nervous system in its distribution. Patients usually present with a headache that is subacute or slowly progressive in onset, severe, and focal or diffuse.48,49 The headache may be accompanied by nausea and vomiting. However, it is usually associated with other neurological manifestations, including hemiparesis, mental impairment, dysphasia, or seizures.50 Symptoms of PACNS may fluctuate in their severity but eventually progress over time. This often leads to delayed diagnosis; as many as 40% of cases are diagnosed after symptoms have been present for more than 3 months.50 Systemic symptoms, such as fever and weight loss, occur much less commonly than with systemic vasculitides. The diagnosis of PACNS can be made through a combination of CSF analysis, angiography, or central nervous system biopsy. CSF analysis often reveals significantly elevated protein levels and white blood cell count in patients with pathologically confirmed PACNS.51 The classic finding on cerebral angiography is a pattern of alternating areas of segmental narrowing and ectasia, producing a beaded or sausage-like appearance. Pathological specimens reveal fibrinoid necrosis and infiltration of vessel walls by lymphocytes, multinucleated giant cells, and/or histiocytes.52 PACNS tends to be an aggressive disease and is uniformly fatal without treatment. Response to cytotoxic/immunosuppressive therapy is variable; remissions are possible in some patients.
RCVS is a unifying diagnosis for a group of disorders characterized by reversible segmental cerebral vasospasm and more benign outcomes than those seen with PACNS. This includes thunderclap headache with vasospasm, benign angiopathy of the central nervous system, migrainous vasospasm or crash migraine, Call-Fleming syndrome, postpartum angiopathy, and drug-induced vasospasm.53–55 Patients with RCVS present with the acute onset of sudden and severe headache, consistent with thunderclap headache. Evaluation reveals normal or near-normal CSF findings and reversible cerebral segmental vasospasm involving arteries of the circle of Willis. The diagnostic criteria for RCVS are (1) a thunderclap headache, (2) evidence of vasospasm of one or more arteries of the circle of Willis that reverses by 12 weeks after onset, and (3) normal or near-normal CSF studies (Table 60-5). Patients may have a history of migraine, may be in the postpartum period, or have had exposure to certain drugs, including ergotamines, triptans, selective serotonin reuptake inhibitors, pseudoephedrine, cocaine, amphetamines, methylenedioxymethamphetamine (ecstasy), or bromocriptine.56–87 Patients with RCVS may differ in regard to the presence and/or severity of neurological deficits, imaging abnormalities, and circumstances at the time of symptom onset. Patients may present with thunderclap headache in isolation or in combination with changes in cognition or consciousness, motor deficits, sensory deficits, seizures, visual disturbances, ataxia, speech abnormalities, nausea, and/or vomiting.
TABLE 60-5 Reversible Cerebral Vasoconstriction Syndrome: Diagnostic Criteria
< div class='tao-gold-member'> Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|