12 Pain Pathophysiology and Management
Neuropathic Pain Syndromes
Pathophysiology
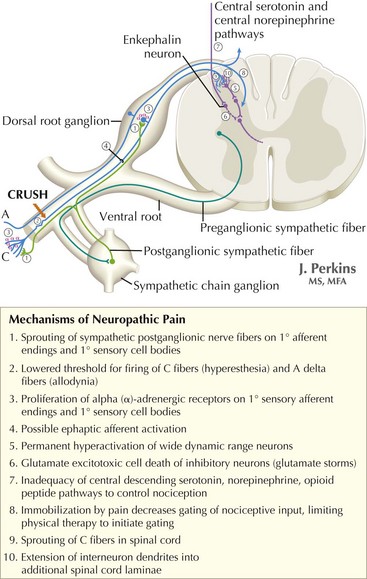
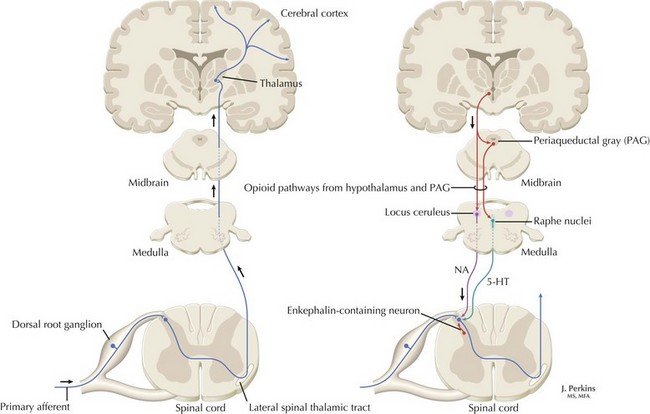
The understanding of the relationship between the clinical features of neuropathic pain and underlying molecular mechanisms in humans remains in its infancy. Following nerve injury, neuronal remodeling occurs, with microscopic structural changes in the neuronal membrane and individual membrane bound ion channels. Animal models suggest that neuronal remodeling alters the membrane electrical properties, resulting in a state of hyperexcitability wherein thresholds are lowered, action potentials are propagated more easily, and the duration of nerve impulses is prolonged. These aberrant action potentials are reproduced at multiple anatomic levels: from the primary sensory neuron, to the sensory ganglia, and neurons within the dorsal horn of the spinal cord (Fig. 12-1). Such a pattern of aberrant nerve discharges may account for the positive symptoms of neuropathic pain. The cognizant perception of painful symptoms is based on the neural pathways commencing in the periphery at the primary sensory nerve ending, transmitted through the dorsal root ganglia, the spinal cord, up to the thalamus and finally the somatosensory parietal cerebral cortex (Fig. 12-2). During the passage of nerve potentials along this pathway, a number of opportunities are available at the various synapses for modulation of the impulses per se and thus the eventual perception of the original stimulus.
Diagnosis and Clinical Manifestations
In addition to the history and physical examination, ancillary studies may aid in diagnosis. These are very important for confirming or excluding the presence of underlying etiologies for neuropathic pain. Magnetic resonance imaging is used to assess the integrity of central neuroanatomic structures involved in pain signaling pathways. These include the spinal cord, brainstem, thalamus, and cortices (see Fig. 12-1). Peripheral sources for the pain can sometimes be defined by electromyography and nerve conduction studies. The latter particularly assess the function of large myelinated nerve fibers. Nonneurologic tests such as oral glucose tolerance, Tzanck prep (a rapid test previously performed to diagnose infections caused by herpes viruses) and enzyme-linked immunosorbent assay. This can be used to confirm or exclude the presence of a number of underlying diseases that may lead to sequelae of neuropathic pain. Psychiatric evaluation is also useful in evaluating possible somatization disorders in patients with multisystem complaints that may date back into early developmental stages.
Treatment
First-Line Prescription Agents
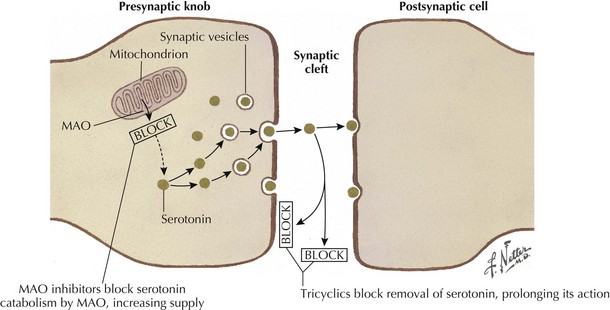
Tricyclic antidepressants historically are thought to modulate pain signaling by affecting both serotonergic and noradrenergic reuptake in descending inhibitory supraspinal pathways (Fig. 12-3). Desipramine and nortriptyline are preferred for neuropathic pain because of their favorable side-effect profiles relative to amitriptyline. Although their anticholinergic side effects demand careful consideration when these are used in patients with comorbidities, TCAs will relieve pain in PHN, postmastectomy pain syndrome, and painful diabetic neuropathy (PDN) and nondiabetic peripheral neuropathy. These agents should be tried alone initially, and then may be used in combination to pursue symptomatic improvement if necessary, provided that the clinician carefully monitors their interactions and side effects.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree