18 Paraneoplastic Disorders
Introduction
Paraneoplastic neurological disorders (PND) are a heterogeneous group of disorders that can affect any part of the neuraxis, including the retina and muscle.1 Unlike other neurological complications that occur in patients with cancer, many PNDs are believed to be mediated by immune mechanisms. The current concept is that the expression of normal neuronal proteins by a cancer induces an immune response that targets the nervous system, resulting in neuronal dysfunction and/or neuronal cell death.2 These immune responses are often associated with the presence of specific antineuronal serum and cerebrospinal fluid antibodies.
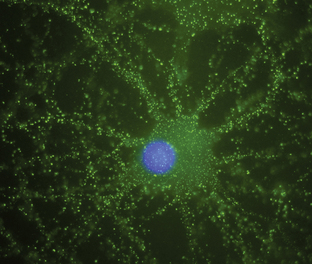
Antineuronal antibodies play a direct pathogenic role in three PNDs that affect the peripheral nervous system. These include antibodies to P/Q-type voltage-gated calcium channels (VGCC) in patients with the Lambert-Eaton myasthenic syndrome (LEMS),3 antibodies to acetylcholine receptor in patients with myasthenia gravis, and antibodies to voltage-gated potassium channels (VGKC) in some patients with peripheral nerve hyperexcitability (neuromyotonia).4 A common feature of these antibodies is that they target cell surface antigens and the associated disorders can occur without cancer; therefore, detection of these antibodies does not predict the presence of cancer. Antibodies to P/Q type VGCC are also found in a subgroup of patients with paraneoplastic cerebellar degeneration (PCD)5 and antibodies to VGKC-related proteins can be found in some patients with cancer-associated or non−cancer-associated limbic encephalitis (LE) and Morvan syndrome.6,7 In these cases, the antibodies are believed to be pathogenic, but this has not yet been proven. Similarly, there is recent evidence that antibodies to the N-methyl-D-aspartate (NMDA) receptor located on the cell surface are associated with a severe form of encephalitis and are likely pathogenic (Figure 18-1).8 An antibody-mediated immunopathogenesis is also strongly suggested for the cerebellar and stiff-person syndromes associated with antibodies to glutamic-acid decarboxylase (GAD), and the paraneoplastic stiff-person syndrome related to antiamphiphysin antibodies. These two antigens are intracellular, close to the synaptic membrane, and the patients’ antibodies appear to have a functional effect in vivo.9,10
For other PNDs, usually those that affect the central nervous system, more complex immune mechanisms appear to exist. In addition to the presence of antineuronal antibodies, PNDs of the central nervous system are associated with infiltrates of CD4+ and CD8+ T cells, microglial activation, gliosis, and variable neuronal loss.11–13 The infiltrating T cells are often in close contact with neurons undergoing degeneration, suggesting a primary pathogenic role. The interaction of B- and T-cell mechanisms and the subacute development of extensive inflammatory abnormalities and neuronal degeneration could explain the difficulty in treating these disorders as well as their poor response to plasma exchange or intravenous immunoglobulins (IVIg).
Although they are increasingly becoming recognized, significant diagnostic delays are frequent even for well-described syndromes. In a series of 50 patients with LEMS, about half of the patients were initially misdiagnosed, usually with myasthenia gravis.14 Another study noted an inverse correlation between the severity of the neurologic symptoms and the time to the diagnosis of the PND.15 For patients who develop a syndrome that is typically associated with cancer and are found to have well-characterized paraneoplastic antibodies, the diagnosis of PND is relatively straightforward. The diagnosis of PND is more difficult in patients who develop less characteristic symptoms, especially if no antibodies are found in the serum or CSF. Features that suggest a paraneoplastic origin include an acute or subacute onset, and, if the central nervous system is involved, the CSF will often suggest an inflammatory process. If the patient is known to have cancer, metastastic or other nonmetastatic complications of cancer should be ruled out. For a patient in cancer remission, a recurrence should be suspected if symptoms of PND develop. For patients without a known cancer, if a PND is suspected, a detailed search for an underlying neoplasm is mandated. Whole body FDG-PET scans may detect tumors that escape detection by other standard imaging methods.16–18 Features of individual syndromes that may aid in diagnosis (e.g., by neuroimaging) are noted in the following descriptions of individual syndromes.
Paraneoplastic Syndromes of the Brain
PARANEOPLASTIC CEREBELLAR DEGENERATION (PCD)
Paraneoplastic cerebellar degeneration is characterized by the rapid development of severe pancerebellar dysfunction that may be preceded by prodromic symptoms including dizziness, oscillopsia, blurry or double vision, nausea, and vomiting. Eventually, symptoms progress to truncal and appendicular ataxia, dysarthria, and downbeating nystagmus.19 Symptoms of brainstem dysfunction, upgoing toes, or a mild neuropathy may occur. The subacute onset of PCD differentiates it from chronic degenerative diseases involving the cerebellum. Early MRI studies are usually normal; in some patients, transient enhancement of the cerebellar cortex has been noted. MRI studies late in the course usually show cerebellar atrophy.
The tumors more frequently involved are small cell lung cancer (SCLC), cancer of the breast and ovary, and Hodgkin lymphoma.20 The paraneoplastic antibodies typically associated with prominent or pure cerebellar degeneration are anti-Yo antibodies in patients with breast and gynecologic cancers, and anti-Tr antibodies in patients with Hodgkin lymphoma. When PCD occurs in association with paraneoplastic encephalomyelitis (PEM), anti-Hu antibodies are almost always present.21 When neoplasms other than breast and gynecological tumors are involved, patients are usually anti-Yo negative. Anti-Yo antibodies have been identified in a few male patients with PCD and cancer of the salivary gland, lung, and esophagus.22,23 Patients with predominant truncal ataxia and opsoclonus or other ocular movement abnormalities may have anti-Ri antibodies, in which case the tumor is usually a breast carcinoma or, less frequently, gynecologic, bladder, or SCLC.24,25 Antibodies to P/Q-type VGCC occur in some patients with SCLC and cerebellar dysfunction, although only some of these patients develop LEMS.5 There is a group of patients, usually with SCLC, who harbor two or more antibodies, such as Zic4 and Hu or CRMP5 or all three. Patients who harbor only Zic4 antibodies are more likely to develop cerebellar dysfunction than patients with several antibodies.26
Prompt tumor control, immunosuppressive intervention, or perhaps different pathogenic mechanisms, may explain a number of single case reports describing neurologic improvement after tumor treatment, plasma exchange, IVIg, cyclophosphamide, or steroids.27–29 However, large series of patients with well-defined antibody-positive PCD show that, in general, there is only rare improvement with treatment, if any.
PARANEOPLASTIC ENCEPHALOMYELITIS
Patients with paraneoplastic encephalomyelitis (PEM) develop multifocal involvement of the nervous system, including brain, brainstem, cerebellum, or spinal cord.15,21 Many patients with PEM also have paraneoplastic sensory neuropathy. The clinical features depend on the area(s) predominantly involved, but pathology studies almost always show abnormalities (inflammatory infiltrates, neuronal loss, gliosis) in asymptomatic regions. Several syndromes have been described that may occur alone or in combination. These include cortical encephalitis, that may present as epilepsia partialis continua; limbic and/or brainstem encephalitis, which is discussed in further detail later; cerebellar gait and limb ataxia; myelitis that may cause lower or upper motor neuron symptoms, myoclonus, muscle rigidity, and spasms; and autonomic dysfunction.
Paraneoplastic encephalomyelitis with or without PSN has been reported in association with almost all types of tumors, but the most common is lung carcinoma, particularly SCLC. The most frequently associated antibodies are anti-Hu and anti-CRMP5/CV2; antibodies to amphiphysin and Zic proteins are less frequently reported.15,26,30
All types of PEM except LE respond poorly to treatment. Stabilization or partial neurologic improvement may occur and usually correlates with tumor response to treatment. In a large series of patients with anti-Hu−associated PEM, treatment of the tumor with or without associated immunotherapy was an independent predictor of neurologic improvement or stabilization.15 The roles of plasma exchange, IVIg, and immunosuppression have not been established. Some patients with LE show marked improvement after tumor treatment and immunomodulatory therapies.31,32
LIMBIC ENCEPHALITIS
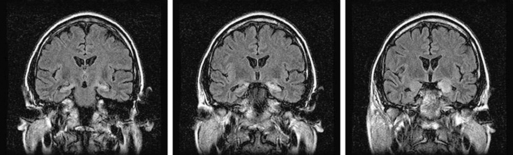
Limbic encephalitis is characterized by confusion, depression, agitation, severe short-term memory deficits, partial-complex seizures, sleep disturbances, and dementia.31 The EEG usually reveals foci of epileptic activity in one or both temporal lobes, or focal or generalized slow activity. About 80% of patients have MRI fluid-attenuated inversion recovery (FLAIR) or, in T2 sequences, hyperintense signal abnormality in the medial aspect of one or both temporal lobes (Figure 18-2). FDG-PET may show hypermetabolism in one or both temporal lobes even when the MRI is normal.33 Recent studies have shown that immune-mediated LE can be categorized into four groups based on the type and location of the target antigens.
Limbic encephalitis associated with antibodies to intracellular antigens
The main intracellular antigens related to LE are Hu, Ma2, and, less frequently, CV2/CRMP5 and amphiphysin. In these immune responses, cytotoxic T cell mechanisms are considered the main pathogenic effectors. Patients with Hu antibodies have PEM, although the disorder may initially present as a focal syndrome; the associated tumor is almost always a SCLC.15,21
Antibodies to Ma proteins are associated with limbic and brainstem encephalitis and occasionally with cerebellar symptoms; prominent hypothalamic dysfunction, hypersomnia, and cataplexy can occur.32,34 Patients less than 50 years of age with limbic dysfunction and antibodies to Ma proteins usually have an underlying germ cell tumor of the testis.35 These patients often benefit from orchiectomy and from immunotherapy that may include corticosteroids and IVIg. Overall, 35% of patients with anti-Ma2 encephalitis have neurological responses to treatment.36 One case of spontaneous neurological improvement has recently been reported.37
Anti-CV2 or CRMP5 antibodies associate with encephalomyelitis, sensorimotor neuropathy, and, more distinctively, with cerebellar ataxia, chorea, uveitis, and optic neuritis.30,38,39 The development of myelitis and optic neuritis may resemble Devic syndrome.40 SCLC and thymoma are the tumors more frequently involved. In patients with SCLC, anti-CV2/CRMP5 may coexist with anti-Hu or Zic antibodies; these patients usually have multifocal deficits or encephalomyelitis.26
Anti-N-methyl-D-aspartate (NMDA) receptor-associated encephalitis
Anti-NMDA receptor-associated encephalitis is a recently described disorder that usually affects young women.8 About 65% of patients have an underlying tumor, usually a cystic teratoma of the ovary. After prodromal symptoms that may include headache, fever, or a viral-like illness, patients develop severe psychiatric symptoms or memory loss, seizures, and decreased level of consciousness, accompanied by dyskinesias, hypoventilation, or autonomic instability. Intensive care support and ventilation may be required for several weeks or many months. Although the disorder is potentially lethal, most patients recover after immunotherapy; when a tumor is found, removal expedites recovery and decreases relapses.41 The disorder can also occur in men or women without a detectable tumor.42 Due to the location of the target antigens on the cell surface (Figure 18-1) and the dramatic response to immunotherapy, it is likely that these antibodies play a direct pathogenic role.
Encephalitis and antibodies to voltage-gated potassium channels
Recent evidence suggests that the target of these antibodies is not in fact the VGKC but other related proteins. The two main syndromes associated with these antibodies include typical LE and a lesser focal encephalitis that is associated with psychiatric symptoms, hallucinations, peripheral nerve hyperexcitability, hyperhydrosis, and other symptoms of autonomic dysfunction (Morvan syndrome). REM sleep disturbances and hyponatremia are common in both, and some patients may develop hypothermia, hypersalivation, pain, and disorders of appetite.43 About 20% of patients with antibodies to VGKC-related proteins have a tumor, often SCLC or thymoma. About 80% of patients will respond to treatment that includes corticosteroids, plasma exchange, or IVIg.
Antibodies to other cell membrane antigens
There are other antibodies to cell surface antigens that have not been fully characterized. Some of these antibodies occur along with other well-characterized immune responses, such as GAD antibodies, and the associated disorders respond differently to immunotherapy.33 It is unclear whether these novel antibodies have one or several target antigens. Tumors found in association with these antibodies include thymoma, SCLC, and Hodgkin lymphoma.
PARANEOPLASTIC OPSOCLONUS-MYOCLONUS
Opsoclonus is a disorder of eye movement characterized by spontaneous, arrhythmic, large-amplitude conjugate saccades occurring in all directions of gaze. Opsoclonus frequently associates with myoclonus and ataxia of the head, trunk, or limbs. When paraneoplastic in adults, symptoms can range from opsoclonus with mild truncal ataxia to a severe syndrome associated with encephalopathy that can lead to stupor and death. A number of associated tumors have been reported, but the most common is SCLC.44 Paraneoplastic opsoclonus-myoclonus in children usually has a subacute onset with frequent fluctuations and is accompanied by ataxia, hypotonia, and irritability.45 Almost 50% of children with paraneoplastic opsoclonus-myoclonus have neuroblastoma, and in half of the patients, the neurologic symptoms precede the diagnosis of the tumor. Children with neuroblastoma and opsoclonus have a better tumor prognosis than those without paraneoplastic symptoms.
Some adult patients, in particular those with SCLC, and 5% to 10% of children with neuroblastoma have anti-Hu antibodies.46 Patients with breast and gynecologic cancers may harbor anti-Ri antibodies;25 some of these patients develop muscle rigidity, autonomic dysfunction, and dementia. A small number of patients have been reported with other antibodies including antibodies to CRMP5/CV2, Zic2, amphiphysin, Yo, and Ma2.30,47,48 However, in many adults and children with neuroblastoma, no paraneoplastic antibodies are found.
When associated with neuroblastoma, the disorder frequently responds to treatment of the tumor, steroids, ACTH, IVIg, plasma exchange, or rituximab;49,50 however, developmental and neurologic sequelae are frequent.45 Paraneoplastic opsoclonus-myoclonus in adults may respond to immunosuppression and IVIg. Patients whose tumors are treated promptly appear to have a better prognosis than those whose tumors are not treated.51
Paraneoplastic Disorders of the Visual System
Paraneoplastic retinopathy is characterized by photosensitivity, progressive loss of vision and color perception, central or ring scotomas, and night blindness.52 The fundoscopic examination is normal or demonstrates arteriolar narrowing, and the electroretinogram (ERG) shows attenuation of photopic and scotopic responses. Paraneoplastic retinopathy associated with antibodies to recoverin is known as cancer-associated retinopathy (CAR).53 Patients with CAR usually have SCLC, but cases have been reported associated with breast or gynecological cancers. Other target antigens that have been described include the tubby-like protein, photoreceptor cell-specific nuclear receptor, and the polypyrimidine tract binding-like protein.54,55 Retinopathy in association with metastatic cutaneous melanoma is known as melanoma-associated retinopathy (MAR).56 As opposed to CAR, these patients present with acute visual loss years or months after the diagnosis of the metastatic disease. The ERG shows reduced or absent b-waves with normal dark-adapted a-waves indicating bipolar cell dysfunction. Some of these patients have antibodies that target unknown antigens in the retinal bipolar cells.57
Optic neuritis has been described in some patients with paraneoplastic syndromes of the central nervous system in association with several antibodies including anti-Hu, anti-Tr, anti-Yo, and, more frequently, anti-CV2/CRMP5.39,58 Patients present with sudden bilateral loss of vision, swollen optic discs, and field defects; the majority have SCLC.
Bilateral diffuse uveal melanocytic proliferation is a rare paraneoplastic entity in which an underlying tumor causes diffuse bilateral proliferation of melanocytes in the uveal tract, leading to bilateral visual loss.59,60 The visual symptoms precede the diagnosis of a systemic malignancy. Carcinoma of the reproductive tract in women and carcinomas of the lung and pancreas in men appear to be the more commonly-associated tumors. Patients present with abrupt bilateral visual loss and few or no findings on examination of the fundus. Nearly all patients described have had rapid cataract progression, and all have had retinal detachment.59 One case ascribed improvement in vision to treatment with external beam irradiation and subretinal fluid drainage.
In general, paraneoplastic visual loss is usually irreversible. Immunosuppression, plasma exchange, or steroids is mostly ineffective but in rare cases may result in symptom stabilization.61
Paraneoplastic Syndromes of the Spinal Cord and Dorsal Root Ganglia
PARANEOPLASTIC MOTOR NEURON SYNDROMES
A wide range of spinal cord syndromes including upper or lower motor neuron dysfunction, myelitis, myelopathy, and sensory and motor neuronopathies have been described in patients with cancer, and it is unclear if these are truly paraneoplastic or simply represent a coincidental association with cancer. Furthermore no specific paraneoplastic antibodies have been found in these patients. A recent study examined the sera of 145 patients with motor neuron disease for well characterized paraneoplastic antibodies (Hu, Yo, Ri, CV2/CRMP5, Ma2 and amphiphysin) and found only low reactivity in five sera that likely represented background activity.62 For some syndromes, such necrotizing myelopathy, the identification of nonparaneoplastic causes such as human herpesvirus has, in many instances, clarified the nature of the disorder.63
The existence of paraneoplastic motor neuron dysfunction is based on reports of patients with typical amyotrophic lateral sclerosis (ALS) who improved after treatment of the underlying tumor (usually renal cell cancer and carcinoma of the lung or breast) suggesting more than a coincidental relationship.64–66 A patient with renal cell carcinoma, neuromyotonia, and lower motor neuron syndrome had recovery of neurological deficits after tumor removal.67 For these patients the neurologic syndrome and laboratory studies are similar to those seen in typical ALS patients. A more-than-coincidental association has been suggested between lymphoproliferative disorders with motor neuron dysfunction.64,68 Patients with PEM may develop symptoms resembling motor neuron disease.21,69 These patients almost always develop signs of involvement of other areas of the nervous system, which, along with the presence of the anti-Hu antibody, helps to rule out typical ALS.
Some patients with cancer develop a subacute lower motor neuronopathy characterized by subacute, progressive, painless, and asymmetrical muscle weakness that is more prominent in the lower extremities.70 Reflexes are decreased or abolished, and, in contrast to typical ALS, bulbar muscles are usually spared, fasciculations are rare, and upper motor neuron signs are absent. Sensory symptoms, if any, are mild and transitory. The neurologic symptoms may have a benign course, independent of the activity of the neoplasm. The associated tumors are Hodgkin lymphoma and less frequently non-Hodgkin lymphoma. This disorder needs to be differentiated from the lower motor neuron syndrome that patients may develop secondary to radiation therapy of the spinal cord.71 In these patients, the distribution of muscle weakness is more distal and, although symptoms stabilize, they do not improve. Patients with Hodgkin lymphoma treated with mantle radiation may develop slowly progressive (over years) weakness and atrophy involving neck flexors and extensors and proximal muscles of the upper extremities. Characteristically, a strip of atrophy involving paraspinal muscles is also observed. Distal reflexes are usually preserved; sensation is normal. No effective therapies have been described.
A disorder with prominent upper motor neuron dysfunction that mimics primary lateral sclerosis has been reported in a few patients with breast cancer. Because no specific paraneoplastic markers have been identified, the association of these disorders may be coincidental.72
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree