CHAPTER 94 PARASITIC AND FUNGAL INFECTIONS
PARASITIC INFECTIONS OF THE CENTRAL NERVOUS SYSTEM
In 1909 John D. Rockefeller declared, “Deprivation in the agriculturally rich Southern States is not due to stupidity or laziness, but to parasite infestation.”1 The human immunodeficiency virus pandemic and global warming have resurrected the study of parasitology. In general, parasitic diseases are treatable and should be considered when geography, patient susceptibility, and exposure make infection possible.
More than 2 million people die each year of falciparum malaria; 200 million are infected with schistosomiasis. Toxoplasma species flourish in patients with acquired immunodeficiency syndrome (AIDS). Epilepsy from neurocysticercosis affects more than 1% of the population in some regions. Most cases of neurocysticercosis in Mexico remain undiagnosed despite the people having cognitive and psychiatric problems throughout life. Sleeping sickness affects 300,000 Africans and threatens many more. Intestinal helminths dull children’s minds.2
This chapter addresses the major neuroparasites, as is customary in the neurosciences, by location and clinical presentation. The aim is to present the clinician with a catalog of possibilities so that a treatable disease is not overlooked (Table 94-1).
TABLE 94-1 Clinical Findings Associated with Neuroparasites
CSF, cerebrospinal fluid; MRI, magnetic resonance imaging.
Amebic Encephalitis
Various features of primary amebic meningoencephalitis (a fulminant encephalitis) and granulomatous amebic encephalitis (a subacute granulomatous disease) are summarized in Table 94-2.
TABLE 94-2 Central Nervous System Amebiasis
| Feature | PAM | GAE |
|---|---|---|
| Risk factors | Swimming | Diabetes, pregnancy, alcohol/cirrhosis, corticosteroids, AIDS, chemotherapy/radiotherapy |
| Organism | Naegleria | Acanthamoeba27,28 |
| Balamuthia | ||
| Route to CNS | Olfactory epithelium/nerves | Intranasal/intracranial vasculitis leading to thrombosis and infarction |
| CNS disease | Culture-negative, fulminant, purulent meningitis; mostly PMN, unlike TB or viral | Focal or diffuse encephalitis with meningism, giant cell reaction29 |
| Organism can harbor | Yes30 | Yes |
| Legionella, Vibrio cholerae | ||
| CSF laboratory findings | Glucose variable; protein >1 g/L | Glucose variable |
| CSF microscopic findings | Motile Naegleria move 1-3 body lengths/min11 | Lymphocytic pleocytosis; trophozoite seldom found in CSF31,32 |
AIDS, acquired immunodeficiency syndrome; CNS, central nervous system; CSF, cerebrospinal fluid; GAE, granulomatous amebic encephalitis; PAM, primary amebic meningoencephalitis; PMN, polymorphonuclear neutrophils; TB, tuberculosis.
Entamoeba histolytica
Diagnosis
Diagnosis is by use of microscopy after staining tissue with Gomori trichrome or the iron hematoxylin method. The sensitivity of microscopy is low. The organism may not be found in stool. Enzyme-linked immunosorbent assay (sensitivity, 87%) and polymerase chain reaction (sensitivity, 85%) are used on stool or serum.34–36
African Trypanosomiasis
Epidemiology
David Livingstone first attributed nagana to the tsetse fly (Glossina) in 1857. The disease is transmitted by male and female tsetse flies through a bite that is painful and does not go unnoticed. Trypanosoma brucei gambiense is found in West Africa; the infection takes months or years to affect the central nervous system (CNS). Trypanosoma brucei rhodesiense causes an acute illness in East Africa, affecting the brain within a few months of the original bite. Transplacental transmission also occurs.
Diagnosis
T. b. rhodesiense, however, can be found in blood. Concentration techniques are used for blood and cerebrospinal fluid (CSF) samples. Inoculation of rodents, which are killed after 21 days, is useful for identification of T. b. rhodesiense, but serological results are positive only after the onset of clinical symptoms, limiting its use in the asymptomatic traveler who is bitten in an endemic area where cattle are dying.37
Treatment
If CSF is positive for leukocytes and IgM concentrations are high, melarsoprol (a trivalent arsenic compound) is the drug of choice (Table 94-3). Relapse rates are high, and up to 20% of patients infected with T. b. gambiense do not respond to melarsoprol. Eflornithine is an alternative therapy for infections from T. b. gambiense but not from T. b. rhodesiense. Melarsoprol may cause post-treatment reactive encephalopathy in up to 10% of cases, with a mortality rate of 50%. Thus, the overall mortality from melarsoprol is 5% from arsenic encephalopathy.
TABLE 94-3 Treatment of Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense Sleeping Sickness*
| Stage | West African (T. b. gambiense) | East African (T. b. rhodesiense) |
|---|---|---|
| Early stage | ||
| Endemic countries | According to national legislation or guidelines | According to national legislation or guidelines |
| Other countries | Pentamidine isethionate, 4 mg/kg body weight daily or on alternate days for 7 to 10 days intravenously† or intramuscularly | Suramin, test dose of 4-5 mg/kg body weight at day 1, followed by 5 injections of 20 mg/kg body weight every 7 days (e.g., days 3, 10, 17, 24, 31); the maximum dose per injection is 1 g |
| Late stage | ||
| Endemic countries | According to national legislation or guidelines | According to national legislation or guidelines |
| Other countries | If available: eflornithine, 100 mg/kg body weight at 6-hour intervals for 14 days (150 mg/kg body weight in children) by short infusions over a period of at least 30 minutes | Eflornithine not recommended (low efficacy38) |
| Alternative: melarsoprol,‡ 3 series of 3.6 mg/kg body weight at 24-hour intervals for 3 days; the series are spaced by intervals of 7 days | Melarsoprol,‡§ 1 series of 1.8, 2.16, 2.52 mg/kg body weight at 24-hour intervals; 1 series of 2.52, 2.88, 3.25 mg/kg body weight at 24-hour intervals; and 1 series of 3.6, 3.6, 3.6 mg/kg body weight at 24-hour intervals; the series are spaced by intervals of 7 days (the maximum dose is 5 mL) | |
* No firm recommendations exist for the use of the trypanocidal drugs; the schedules indicated are those most commonly used. A concise treatment schedule for treatment of T. b. gambiense sleeping sickness consisting of 10 days of melarsoprol, 2.2 mg/kg body weight daily, is under final evaluation.39,40 Note: This 10-day schedule must not be used for treatment of T. b. rhodesiense sleeping sickness, because there are no data available.
† Very slow intravenous injection or short infusion, only under well-controlled circumstances.
‡ The concomitant application of 1 mg/kg body weight prednisolone has been shown to reduce the incidence of encephalopathic syndromes in one large-scale clinical trial.41
§ A single dose of Suramin is often applied before the stage determining lumbar puncture.
From Burri C, Brun R: Human African trypanosomiasis. In Cook GC, Zumla AI, eds: Manson’s Tropical Diseases, 21st ed. Edinburgh: Saunders, 2003, pp 1303-1323. Used with permission.
A lumbar puncture should be performed 1 day after a course of therapy for late-stage disease. All patients should be monitored for 2 years with lumbar punctures every 6 months. A relapse is suspected if the CSF cell count is more than 20 cells/μL. Reinfection is likely when CSF has more than 50 cells/μL, when the count has doubled since the previous count, or when there are 20 to 49 cells/μL in a symptomatic patient.25,39
Angiostrongylus cantonensis
Epidemiology
Angiostrongylus cantonensis is found mostly in southeast Asia and the Pacific Basin, but its distribution is spreading to Africa and the Caribbean. The infection is mainly acquired by eating raw or undercooked snails or slugs; it may also be acquired from eating crabs and freshwater shrimp.
Clinical Presentation
This worm, more than Gnathostoma or Toxocara, is the main cause of eosinophilic meningitis worldwide (Table 94-4). Larvae in the brain cause headache, vomiting, neck stiffness, seizures, and focal neurological signs.
TABLE 94-4 Causes of Cerebrospinal Fluid Eosinophilia
Cysticercus of Taenia solium
Epidemiology
Humans are an accidental end-stage host of cysticerci, which die and degenerate in the brain, eye, and spinal cord, causing an inflammatory reaction. Cysticercosis is the most common cause of epilepsy in developing countries. T. solium intestinal carriers, releasing up to 200,000 eggs per day into their environment, are infective sources of cysticercosis, endangering all who come in contact with them. In 1993, the World Health Organization declared cysticercosis a potentially eradicable disease by tracing human carriers of T. solium. In the early 19th century, cysticercosis was diagnosed in 2% of autopsies in Berlin, roughly the same rate as found today in Mexico.11 The Commission on Tropical Diseases of the International League Against Epilepsy states that the prevalence of active epilepsy in tropical countries ranges from 1% to 1.5%, almost twice the level in Western countries. Regions and peoples with a religious aversion to pork are spared.24,43,44
Clinical Presentation
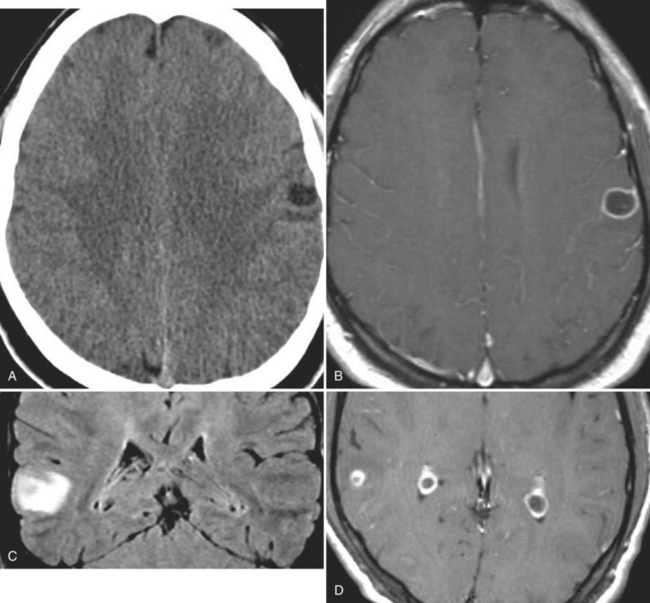
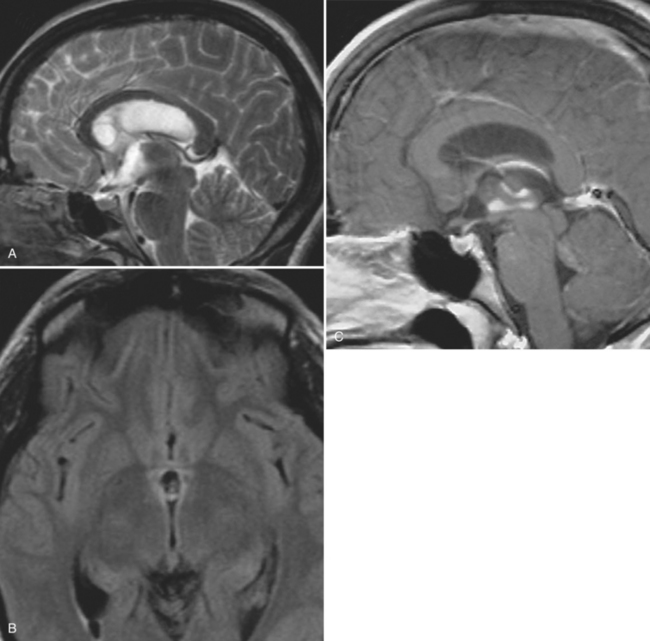
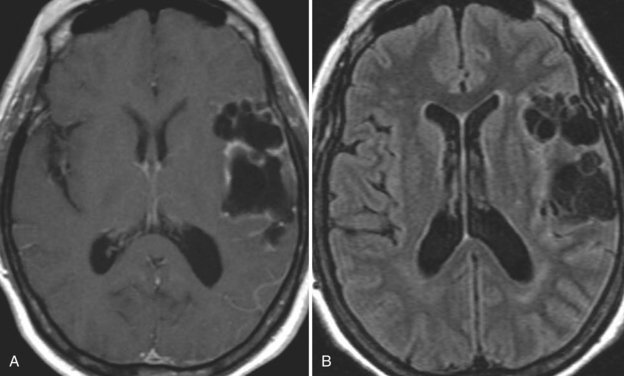
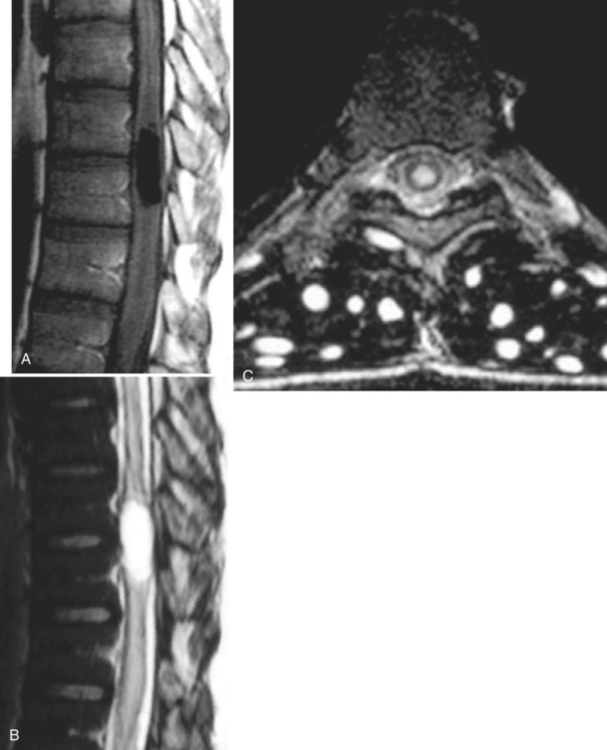
With parenchymal disease, most children (80%) present with seizures. Episodic headaches and vomiting are featured in one third. Ventricular cysts may wander in the isodense CSF and cause hydrocephalus through an intermittent ball-valve effect. Subarachnoid cysts can result in meningeal signs, usually without fever. Rarely, cysticerci erode into large vessels. Arachnoiditis is another cause of hydrocephalus, as well as focal vasculitis, which may be responsible for lacunar strokes. Small space-occupying lesions are responsible for focal weakness. Cognitive and psychiatric impairments are common. In the eye, a scotoma may develop or vision may be lost.45
Diagnosis
Blood and CSF serological analyses have improved. Many clinicians use the enzyme-linked immunoelectrotransfer blot (EITB) assay. It derives from coproantigens of adult T. solium and ignores nonspecific bands of antigen. EITB results may revert to negative after the cysticercus dies. The assay is not always readily available. In the blood, sensitivity is 92.5% and specificity is 100%. However, EITB sensitivity has been reported to be as low as 28% in subjects with a single parenchymal lesion, as is commonly seen in India.46 EITB sensitivity may be less in CSF.47
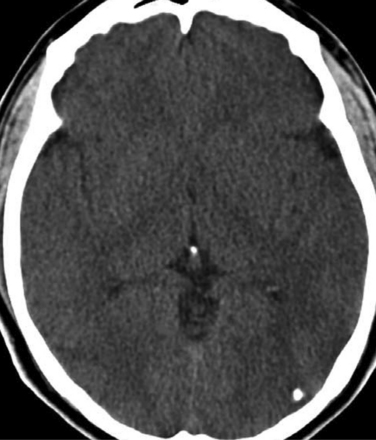
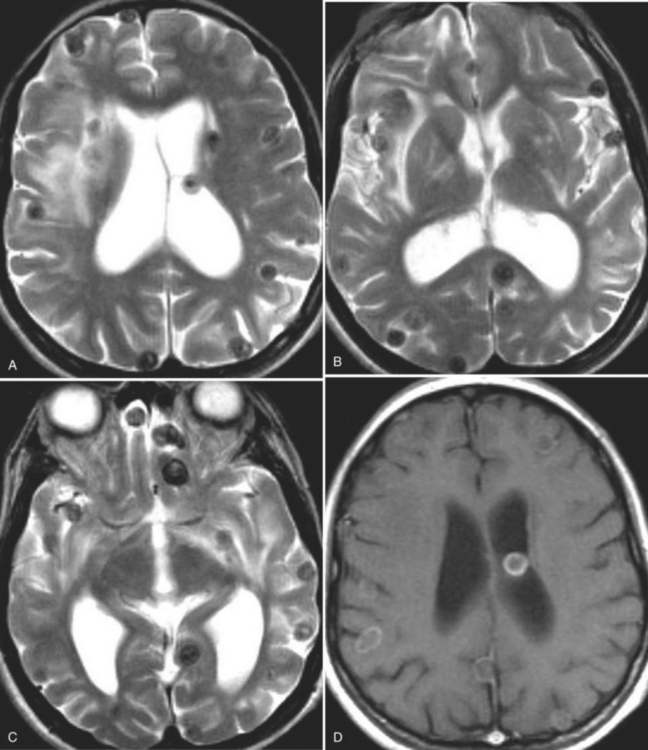
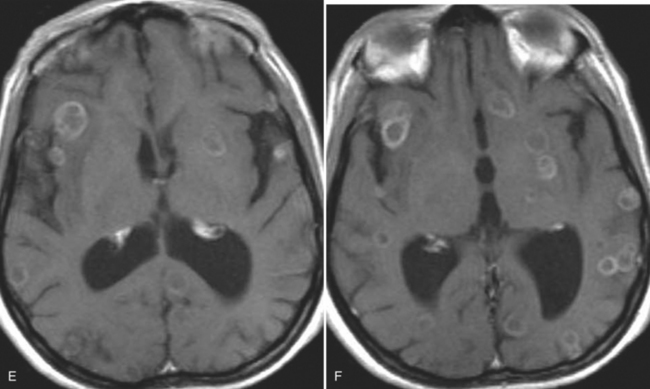
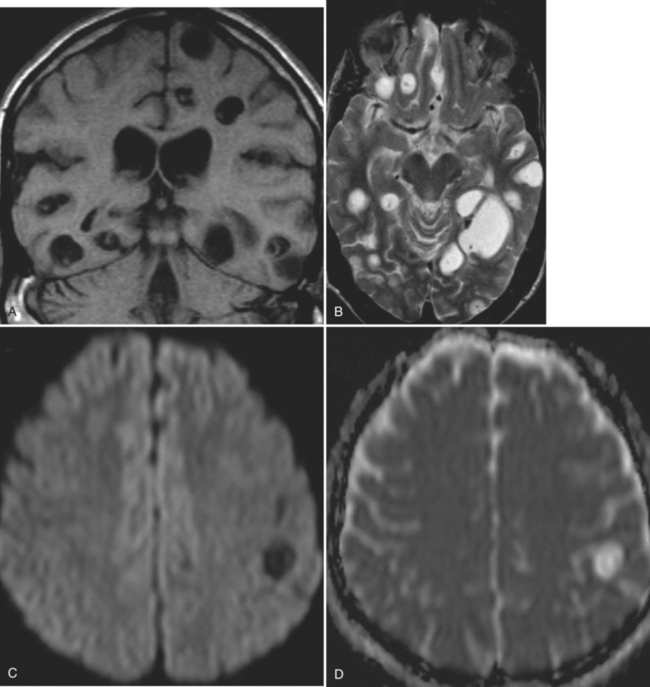
The diagnosis requires neuroimaging, with the goal of identifying the scolex (2 to 3 mm). Only since the frequent use of computed tomography in the 1980s has cysticercosis emerged as the most common cause of epilepsy in many areas. (Cerebral malaria is likely the most common cause of febrile seizures.) Magnetic resonance imaging may show a diagnostic invaginated scolex but not show the calcific stage, for which computed tomography is best. Magnetic resonance imaging can show ventricular cysts. In brain parenchyma, the cysts mature in 3 months to 10mm in size (occasionally up to 20mm); in the ventricles, they can exceed 5 cm. Therefore, serum EITB, CSF ELISA, and neuroimaging have become useful diagnostic tools48,49 (Figs. 94-1 through 94-7).
Treatment
 In the tropics, studies have shown that hypoproteinemia, malabsorption, and malnutrition affect drug kinetics. This has been shown with chloroquine, phenylbutazone, chloramphenicol, carbamazepine, and others. Higher doses of antiepileptics are sometimes required (Fig. 94-8).
In the tropics, studies have shown that hypoproteinemia, malabsorption, and malnutrition affect drug kinetics. This has been shown with chloroquine, phenylbutazone, chloramphenicol, carbamazepine, and others. Higher doses of antiepileptics are sometimes required (Fig. 94-8).Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree