CHAPTER 72 PARKINSON PLUS DISORDERS
The term parkinson plus disorders has been coined to embrace a heterogeneous group of movement disorders with prominent signs of parkinsonism plus additional features that allow clinical separation of these entities from classic idiopathic Parkinson disease (IPD). A synonymous designation is “atypical parkinsonian disorders” where the prefix “atypical” again refers to features that either are not part of the clinical spectrum of IPD—such as cerebellar ataxia, pyramidal tract signs, myoclonus, supranuclear gaze palsy, or apraxia—or are more pronounced and/or occur earlier in the disease course compared with IPD—such as autonomic failure or dementia. Poor or absent responses to L-dopa is another criterion of “atypical” parkinsonian disorders (APDs).
MULTIPLE SYSTEM ATROPHY
MSA is a sporadic neurodegenerative disorder characterized clinically by any combination of parkinsonian, autonomic, cerebellar, or pyramidal symptoms and signs and pathologically by cell loss, gliosis, and glial cytoplasmic inclusions (GCIs) in several brain and spinal cord structures. The term multiple system atrophy was introduced in 19691; however, cases of MSA were previously reported under the rubrics of striatonigral degeneration (SND),2–4 olivopontocerebellar atrophy (OPCA),5,6 Shy-Drager syndrome (SDS),7 and idiopathic orthostatic hypotension (OH). In 1989, GCIs were first described in the brains of patients with MSA regardless of clinical presentation.8 GCIs were not present in a large series of patients with other neurodegenerative disorders. The abundant presence of GCIs in all clinical subtypes of MSA led to the recognition of SDS, SND, and sporadic OPCA as one disease entity characterized by neuronal multisystem degeneration with unique oligodendroglial inclusion pathology. In the late 1990s, α-synuclein immunostaining was recognized as a sensitive marker of inclusion pathology in MSA,9,10 and MSA is now classified among the “synucleinopathies” along with Parkinson disease and dementia with Lewy bodies (DLBs).
Epidemiology
There are only few descriptive epidemiological studies on MSA. Bower and colleagues reported the incidence of MSA over a 14-year period in Olmsted County, Minnesota. Nine incident cases of MSA were identified, none of which had an onset before the age of 50 years. The reported crude incidence rate was 0.6 case per 100,000 population per year; when the age band of greater than 50 years was examined, the estimate rose to 3 cases per 100,000 population. Estimates of the prevalence of MSA (per 100,000 in the population) in four studies ranged from 1.9 to 4.9.11–14 These prevalence figures are similar to those of other well-known neurodegenerative disorders such as Huntington disease and motor neuron disease. Although many studies report a possible role of environmental toxins in Parkinson disease, such a role is even more likely in MSA, as this is a sporadic disease. However, to date only three studies have addressed environmental risk factors in MSA.15–17 So far, no single environmental factor has been clearly established as conferring increased or reduced risks to develop MSA.
Clinical Presentation, Course, and Prognosis
The disease affects both men and women, it usually starts in the sixth decade, and it relentlessly progresses with a mean survival of 6 to 9 years.18–21 There is considerable variation of disease progression, with survival of longer than 15 years in some instances.
Clinically, cardinal features include autonomic failure, parkinsonism, cerebellar ataxia, and pyramidal signs in any combination. Previous studies suggest that 29% to 33% of patients with isolated late-onset cerebellar ataxia and 8% of patients presenting with parkinsonism eventually develop MSA.22–24 Two major motor presentations can be distinguished clinically. Parkinsonian features predominate in 80% of patients (MSA-P subtype), and cerebellar ataxia is the major motor feature in 20% of patients (MSA-C subtype).21,25 Importantly, both motor presentations of MSA are associated with similar survival times.20 However, MSA-P patients have a more rapid functional deterioration than MSA-C patients.18
MSA-P–associated parkinsonism is characterized by progressive akinesia and rigidity. Jerky postural tremor and, less commonly, tremor at rest may be superimposed. Frequently, patients exhibit orofacial or craniocervical dystonia (Fig. 72-1)26 associated with a characteristic quivering high-pitched dysarthria. Postural stability is compromised early on; however, recurrent falls at disease onset are unusual, in contrast to progressive supranuclear palsy (PSP). Differential diagnosis of MSA-P and Parkinson disease may be exceedingly difficult in the early stages due to a number of overlapping features such as rest tremor or asymmetrical akinesia and rigidity. Furthermore, L-dopa–induced improvement of parkinsonism may be seen in 30% of MSA-P patients. However, the benefit is transient in most of these subjects, leaving 90% of the MSA-P patients unresponsive to L-dopa in the long term. L-Dopa–induced dyskinesia affecting orofacial and neck muscles occurs in 50% of MSA-P patients, sometimes in the absence of motor benefit.27 In most instances, a fully developed clinical picture of MSA-P evolves within 5 years of disease onset, allowing a clinical diagnosis during follow-up.28
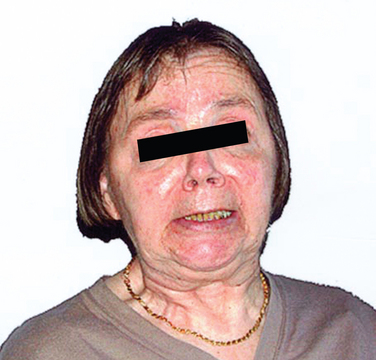
Figure 72-1 Orofacial dystonia in MSA.
(From Wenning GK, Geser F, Poewe W. The ‘risus sardonicus’ of multiple system atrophy. Mov Disord 2003; 18:1211.)
The cerebellar disorder of MSA-C comprises gait ataxia, limb kinetic ataxia, and scanning dysarthria as well as cerebellar oculomotor disturbances. Patients with MSA-C usually develop additional noncerebellar symptoms and signs but before doing so may be indistinguishable from other patients with idiopathic late-onset cerebellar ataxia, many of whom have a disease restricted clinically to cerebellar signs and pathologically to degeneration of the cerebellum and olives.22
Dysautonomia is characteristic of both MSA motor presentations, primarily comprising urogenital and orthostatic dysfunction. Early impotence (erectile dysfunction) is virtually universal in men with MSA, and urinary incontinence or retention, often early in the course or as presenting symptoms, is frequent.21 Disorders of micturition in MSA are due to changes in the complex peripheral and central innervation of the bladder29 and generally occur more commonly, earlier, and to a more severe degree than in Parkinson disease. In contrast, constipation occurs equally in Parkinson disease and MSA. Symptomatic OH is present in 68% of clinically diagnosed patients, but recurrent syncopes emerge in only 15%.21 Levodopa or dopamine agonists may provoke or worsen OH.
Diagnostic Criteria
Clinical diagnostic criteria for MSA were first proposed by Quinn,30,31 who classified cases as either SND or OPCA type MSA depending on the predominance of parkinsonism or cerebellar ataxia.
More recently, operationalized criteria have been proposed by an International Consensus Conference.25 The consensus criteria have since been widely established in the research community as well as movement disorders clinics. They define three diagnostic categories of increasing certainty: possible, probable, and definite. The diagnosis of possible and probable MSA is based on the presence of specific clinical features (Table 72-1). In addition, exclusion criteria have to be considered. A definite diagnosis requires a typical neuropathological lesion pattern with α-synuclein–positive GCIs.
TABLE 72-1 Multiple System Atrophy Consensus Criteria
| Domain | Criterion | Feature |
|---|---|---|
| Autonomic and urinary dysfunction | Orthostatic fall in blood pressure (by 30 mmHg systolic or 15 mmHg diastolic) | Orthostatic hypotension (by 20 mmHg systolic or 10 mmHg diastolic) |
| or | Urinary incontinence or incomplete bladder emptying | |
| persistent urinary incontinence with erectile dysfunction in men | ||
| or both | ||
| Parkinsonism | Bradykinesia plus rigidity | Bradykinesia (progressive reduction in speed and amplitude of voluntary movements during repetitive actions) |
| or | Rigidity | |
| postural instability | Postural instability (loss of primary postural reflexes) | |
| or | Tremor (postural, resting, or both) | |
| tremor | ||
| Cerebellar dysfunction | Gait ataxia plus ataxic dysarthria | Gait ataxia (wide-based stance with irregular steps) |
| or | Ataxic dysarthria | |
| limb ataxia | Limb ataxia | |
| or | Sustained gaze-evoked nystagmus | |
| sustained gaze-evoked nystagmus | ||
| Corticospinal tract dysfunction | No defining features | Extensor plantar responses with hyperreflexia |
Nomenclature of clinical domains, features (disease characteristics), and criteria (defining features or composite of features) used in the diagnosis of multiple system atrophy.
Modified from Gilman S, Sima AA, Junck L, et al: Spinocerebellar ataxia type 1 with multiple system degeneration and glial cytoplasmic inclusions. Ann Neurol 1996; 39: 241-255.
A recent retrospective evaluation of the Consensus criteria on pathologically proven cases showed excellent positive predictive values for both possible and probable MSA; however, sensitivity for probable MSA was poor.32 While such formal diagnostic criteria are important for certain types of clinical research, they add little to the problem of detecting early cases, and improved screening instruments are certainly needed.
Besides the poor response to levodopa, and the additional presence of pyramidal or cerebellar signs or autonomic failure as major diagnostic clues, certain other features (“red flags”) such as orofacial dystonia, stridor, or REM sleep behavior disorder (RBD) may raise suspicion of MSA.30,33 MSA patients may present with isolated RBD.34–36 RBD and other sleep disorders are more common in patients with MSA than in those with Parkinson disease matched for disease duration, reflecting both a profound striatal monoaminergic deficit37 and diffuse subcortical and brainstem disease in MSA.38
Genetics
MSA, as reflected in its current definition, is regarded as a sporadic disease,39 and no confirmed familial cases of MSA have been described; notwithstanding, it is conceivable that genetic factors may play a role in the etiology of the disease. This has, for example, been convincingly demonstrated for PSP,40 another disease that, in the vast majority of cases, is a sporadic disease. However, initial screening studies for candidate genes revealed no risk factors.41,42 Other studies have looked for polymorphisms or mutations in candidate genes, which may predispose an individual toward developing MSA. The apolipoprotein ε4 allele is not overrepresented in MSA when compared with controls, and there have been conflicting reports of the association of a cytochrome P450-2D6 polymorphism with MSA.43,44,45 Furthermore, there is no evidence to support an association between MSA and polymorphisms in the H5 pore region of the human homolog of the weaver mouse gene hiGIRK2, the insulin-like growth factor 1 receptor gene, or the ciliary neurotrophic factor gene.41 Genotyping of a functional polymorphism in the dopamine beta-hydroxylase (DBH) gene showed no association between the DBH–1021 C→T polymorphism and MSA.46 Increased expression of a brain specific protein called ZNF231 in cerebellar neurons has been reported to occur in patients with MSA.47 The gene is located on chromosome 3p21 and encodes a neuronal double zinc finger protein with a nuclear targeting sequence, suggesting that it might function as a transcription regulator. The importance of this finding is as yet uncertain, but it is possible that patients with MSA differ from unaffected individuals by sequence polymorphisms within, and flanking, the putative functional motifs of the ZNF231 gene.
Gilman and colleagues48 reported an MSA-like phenotype including GCIs in one SCA-1 (spinocerebellar ataxia type 1) family. Other SCA mutations, except for SCA-2,27 have not been reported to present with MSA-like features.49–54 Conversely, the majority of MSA-C patients do not appear to have expanded SCA1 and SCA3 alleles.41 Indeed, MSA-C appears to be a frequent form of sporadic cerebellar ataxia of late onset; 29% of sporadic adult-onset ataxia patients suffer from MSA.22 This finding corresponds well with data of a study of sporadic OPCA patients who were followed 3 months to 10 years.23 Within this period, 17 of 51 patients developed autonomic failure or parkinsonism indicating a diagnosis of MSA. There is significant overlap in clinical and radiological features between the fragile X–associated tremor/ataxia syndrome (FXTAS) and atypical parkinsonism, in particular, MSA-C. A considerable number of cases with FXTAS have already been described in male carriers of the fragile X premutation.55,56 Kamm and colleagues found an elevated frequency of fragile X mental retardation 1 gene (FMR1) “gray zone” alleles (40 to 54 repeats) in both male MSA and PSP patients compared with controls, suggesting that small repeat expansion in this gene may possibly act as a susceptibility factor for certain types of neurodegenerative diseases in apparently sporadic male patients, probably in combination with other genetic and environmental factors.57 In contrast, there was no significant difference in allele frequency for either FMR1 “gray zone” alleles for female patients or for FMR1 premutation alleles (55 to 200 repeats) in both male and female patients compared with healthy controls, indicating that FXTAS due to a premutation in the FMR1 gene represents only a rare cause of apparently sporadic atypical parkinsonism.57
Ancillary Investigations
The diagnosis of MSA still rests on the clinical history and neurological examination. According to the Consensus Conference on the diagnosis of MSA,25 additional investigations such as autonomic function tests, sphincter electromyography, and neuroimaging may be used to support the diagnosis or to exclude other conditions. The abnormalities reviewed below have been observed in patients with advanced rather than early disease. In the early stages the investigations may give equivocal results. Therefore, the Consensus Conference on MSA considered it premature to incorporate the results of laboratory investigations into the diagnostic guidelines that were established.
Cardiovascular Function
A history of postural faintness or other evidence of OH, such as neck ache on rising in the morning or posturally related changes of visual perception, should be sought in all patients in whom MSA is suspected. After taking a comprehensive history, testing of cardiovascular function should be performed. According the consensus statement of the American Autonomic Society and the American Academy of Neurology on the definition of OH, pure autonomic failure, and MSA, a drop in systolic blood pressure of 20 mm Hg or more, or in diastolic blood pressure of 10 mm Hg or more, compared with baseline is defined as OH and must lead to more specific assessment.58 This is based on continuous noninvasive measurement of blood pressure and heart rate during tilt-table testing.59 In MSA, cardiovascular dysregulation appears to be caused by central rather than peripheral autonomic failure. During supine rest, norepinephrine levels (representing postganglionic sympathetic efferent activity) are normal,60 and there is no denervation hypersensitivity.61 In contrast, mainly postganglionic sympathetic dysfunction is thought to account for autonomic failure associated with Parkinson disease. However, a study demonstrated that abnormal cardiovascular autonomic function tests failed to differentiate autonomic failure associated with Parkinson disease versus MSA.62 Although such abnormalities may be nonspecific, their presence within the first 3 to 5 years of disease onset make a diagnosis of MSA more likely than Parkinson disease.
Bladder Function
Assessment of bladder function is mandatory in MSA and usually provides evidence of involvement of the autonomic nervous system at an early stage of the disease (when bladder function is still normal in most Parkinson disease patients). Following a careful history regarding frequency of voiding, difficulties in initiating or suppressing voiding, and the presence of urinary incontinence, a standard urine analysis should exclude an infection. Postvoid residual volume needs to be determined sonographically or via catheterization to initiate intermittent self-catheterization in due course. In some patients, only cystometry can discriminate between hypocontractile detrusor function and a hyperreflexic sphincter-detrusor dyssynergy. The nature of bladder dysfunction is different in MSA and Parkinson disease. Although frequency and urgency are common in both disorders, marked urge or stress incontinence with continuous leakage is not a feature of Parkinson disease apart from very advanced cases. Urodynamic studies show a characteristic pattern of abnormality in MSA patients.63 In the early stages there is often detrusor hyperreflexia, often with bladder neck incompetence due to abnormal urethral sphincter function, which results in early frequency and urgency followed by urge incontinence. Later on, the ability to initiate a voluntary micturition reflex and the strength of the hyperreflexic detrusor contractions diminish, and the bladder may become atonic, accounting for increasing postmicturition residual urine volumes.
Sphincter Electromyography
An abnormal sphincter electromyogram (EMG) may be found in many patients with clinically definitive MSA, including those who, as yet, have no urological or anorectal problems. In at least 80% of patients with MSA, an EMG of the external anal sphincter reveals signs of neuronal degeneration in Onuf’s nucleus with spontaneous activity and increased polyphasia.29,64,65 The prevalence of abnormalities in early stages of MSA remains unclear. These findings do not reliably differentiate between MSA and other forms of APD such as PSP.66 Furthermore, neurogenic changes of external anal sphincter muscle have also been demonstrated in advanced stages of Parkinson disease.67 Also, chronic constipation, previous pelvic surgery, or vaginal deliveries can be confounding factors to induce nonspecific abnormalities.68 However, anal sphincter EMG abnormalities appear to distinguish MSA from Parkinson disease in the first 5 years after disease onset, and from pure autonomic failure, as well as from cerebellar ataxias, if other causes for sphincter denervation have been ruled out.69
Imaging
Magnetic Resonance Imaging
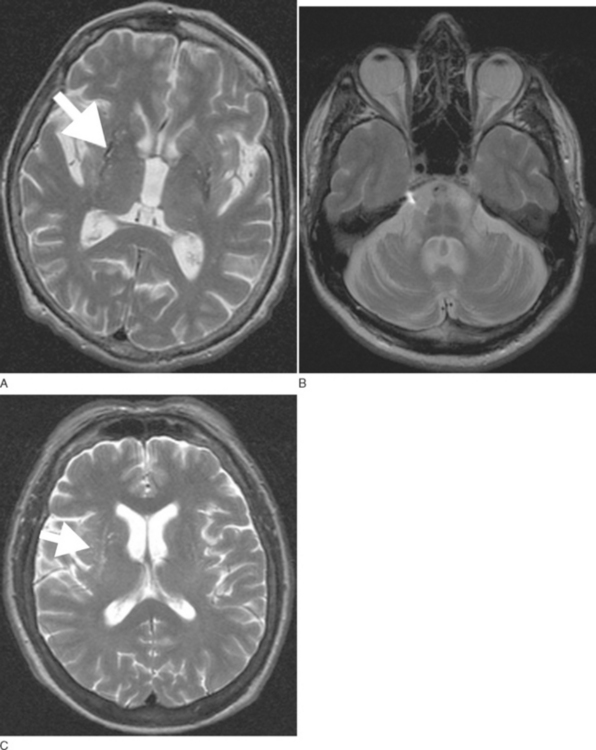
Routine 1.5-T magnetic resonance imaging (MRI) including diffusion weighted imaging (DWI) should be performed in all patients with suspected MSA because basal ganglia and/or brainstem abnormalities suggestive of MSA may be observed even during early disease stages. These changes include an OPCA-like atrophy pattern indistinguishable from autosomal dominant cerebellar ataxia.70 MRI measures of basal ganglia pathology in MSA are less well established, and naked eye assessments are often unreliable. In advanced cases, putaminal atrophy may be detectable and may correlate with severity of extrapyramidal symptoms (Fig. 72-2A, B).
Abnormalities on MRI may include not only OPCA70 or putaminal atrophy71 but also signal abnormalities on T2-weighted images. Signal hyperintensities within the pons and middle cerebellar peduncles are thought to reflect degeneration of pontocerebellar fibers; these changes occasionally resemble a hot cross bun.71 Nonspecific putaminal hypointensities in patients with atypical parkinsonism including MSA were first reported in 1986 by two groups using a 1.5-T T2-weighted images.72,73 This change has subsequently been confirmed by others in cases with pathologically proven MSA.74–76 Similar MRI abnormalities may occur in patients with classic Parkinson disease.77 However, a study demonstrated that hypointense putaminal signal changes were more frequent in MSA than in Parkinson disease patients using T2*-weighted gradient echo (GE) instead of T2-weighted fast spin echo images, indicating that T2*-weighted GE sequences are of better diagnostic value for patients with parkinsonism.78 Increased putaminal hypointensities may be associated with a slit-like hyperintense band lateral to the putamen79,80 (Fig. 72-2C). The latter appears to be more specific for MSA than putaminal hypointensity71,79,80; however, further studies in larger cohorts of patients are needed to confirm this. The hyperintense slit-signal correlated with reactive microgliosis and astrogliosis in a case with pathologically proven MSA.76
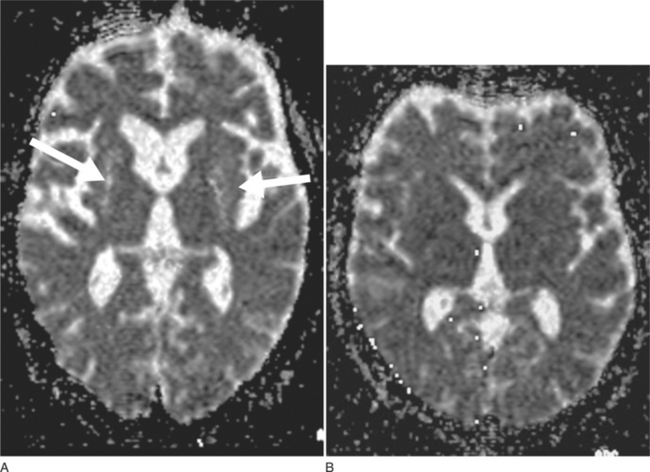
DWI may represent a useful diagnostic tool that can provide additional support for a diagnosis of MSA-P. DWI is able to discriminate MSA-P and both patients with Parkinson disease and healthy volunteers on the basis of putaminal rADC (regional apparent diffusion coefficient) values81 (Fig. 72-3A, B). The increased putaminal rADC values in MSA-P are likely to reflect ongoing striatal degeneration, whereas most neuropathological studies reveal intact striatum in Parkinson disease. But, since in PSP compared with Parkinson disease rADCs were also significantly increased in both putamen and globus pallidus,82 increased putaminal rADC values do not discriminate MSA-P from PSP.
Schulz et al.83 found significant reductions in mean striatal and brainstem volumes in patients with MSA-P, MSA-C, and PSP, whereas patients with MSA-C and MSA-P also showed a reduction in cerebellar volume. More recently, voxel-based morphometry confirmed previous region of interest (ROI)–based volumetric studies83 showing basal ganglia and infratentorial volume loss in MSA-P patients.81 These data also revealed prominent cortical volume loss in MSA-P mainly comprising the cortical targets of striatal projections such as the primary sensorimotor, lateral premotor cortices, and the prefrontal cortex. MR-based volumetry is a helpful tool to investigate the progression of cortical and subcortical atrophy patterns in MSA compared with other disorders; however, it cannot be applied for routine diagnostic workup of individual patients.
Functional Imaging (Single-Photon Emission Computed Tomography and Positron Emission Tomography)
Overall presynaptic and postsynaptic dopaminergic markers have yielded disappointing results regarding the differentiation between Parkinson disease, MSA, and PSP.85 PET studies using other ligands such as [11C]diprenorphine (nonselective opioid receptor antagonist)86 and 18F-fluorodeoxyglucose ([18F]FDG)87–89 have proved more consistent in detecting striatal degeneration and in distinguishing patients with MSA-P from those with Parkinson disease, particularly when combined with a dopamine D2 receptor scan.90 Widespread functional abnormalities in MSA-C have been demonstrated using [18F]FDG and PET.91 Reduced metabolism was most marked in the brainstem and cerebellum, but other areas such as the basal ganglia and cerebral cortex were also involved, supporting its nosological status as the cerebellar subtype of MSA.
SPECT evaluation of the dopamine transporter (DAT) using [123]β-CIT [2β-carboxymethoxy-3β-(4-iodophenyl)tropane] reflects the disruption of the nigrostriatal pathway, and therefore MSA and PSP cannot be separated from Parkinson disease with this method alone.92 However, a study using voxelwise SPM analysis of DAT-binding found significant differences between Parkinson disease and MSA patients in an area including the mesopontine junction, and this type of analysis may enhance the differential diagnostic potential DAT-SPECT in MSA versus Parkinson disease.93
SPECT studies using [123I]iodobenzamide ([123] IBZM) as D2 receptor ligand have revealed significant reductions of striatal IBZM binding in clinically probable MSA subjects compared with Parkinson disease patients or controls.94–96 However, striatal IBZM binding is also reduced in other APDs such as PSP95 limiting its predictive value for an early diagnosis of MSA.
Scintigraphic visualization of sympathetic cardiac neurons using scintigraphy with [123I]metaiodobenzylguanadine ([123I] MIBG) has been shown to reveal loss of binding in patients with Parkinson disease regardless of disease severity, reflecting postganglionic sympathetic denervation compared with preserved cardiac binding in MSA97,98 and PSP.95 Considering all reports published so far, MIBG scintigraphy was able to accurately discriminate a total of 246 Parkinson disease patients from 45 MSA patients with high sensitivity (90%) and specificity (95%).98 Similarly, 18F-dopa PET is able to demonstrate cardiac sympathetic denervation in pure autonomic failure and Parkinson disease in contrast with intact cardiac sympathetic innervation in MSA.99
Principles of Management
Symptomatic Therapy
There is virtually no effective treatment for the cerebellar features of the disease.100,101 Therefore, medical treatment is largely aimed at alleviating parkinsonism and dysautonomia.
Parkinsonism
The commonly held belief that patients with MSA are non–or poorly L-dopa–responsive is misleading. Clinical series have documented levodopa efficacy in up to 40% of patients with possible or probable MSA.21,102–104 Data obtained from series with pathological confirmation are more variable, with rates of beneficial L-dopa response ranging between 30% and 80%.105–108 L-Dopa responsiveness should be tested by administering escalating doses (with a peripheral decarboxylase inhibitor) over a 3-month period up to a least 1000 mg/day (if necessary and if tolerated).25 However, whatever response there is it usually declines after a few years of treatment.31 Dyskinesias emerge in half of the patients treated with L-dopa and they are often dystonic, predominantly affecting the orofacial district.27,105
Results with dopamine agonists are also variable, but these compounds are no more effective than levodopa and often poorly tolerated. Goetz and colleagues,100 using doses of 10 to 80 mg/day of bromocriptine, reported a benefit in five patients who had previously responded to L-dopa and one patient who had failed to respond to levodopa. In a controlled trial with lisuride in seven patients, only one, who had already responded to levodopa, showed improvement.109 No formal trials looking at the efficacy of pergolide, cabergoline, ropinirole, or pramipexole are available.
Despite anecdotal benefit in single cases, a short-term open trial with amantadine at high doses (400 to 600 mg/day) in five patients with MSA unresponsive to L-dopa was negative.110 Anticholinergics usually do not improve motor symptoms, but they may be helpful when sialorrhea is severe and disturbing.
Ablative neurosurgical procedures such as medial pallidotomy fail to improve parkinsonian motor disturbance in MSA.111 However, beneficial short-term and long-term effects of bilateral subthalamic nucleus highfrequency stimulation have been reported in four patients with MSA-P.112 Further studies are needed to establish the scope of deep brain stimulation in MSA. Alternative therapeutic neurosurgical strategies such as trophic factors local delivery and neurotransplantation are currently being explored experimentally in animal models of MSA.113
Autonomic Dysfunction
Treatment of OH is often fraught with difficulties, but it is crucial to improve the quality of life in patients with MSA and autonomic dysfunction.114 The treating physician should not to be excessively concerned about a low standing blood pressure if the patient is asymptomatic. Patients with MSA can sometimes tolerate a decreased standing systolic blood pressure without symptoms, probably because their cerebral blood flow is kept at an adequate level thanks to a functioning autoregulation.115 The latter appears to be preserved down to a systolic blood pressure of 60 mm Hg, well below the 80 mm Hg at which autoregulation fails in normal subjects.116
When OH becomes disabling, it can often be alleviated by progressively avoiding aggravating factors, such as the effects of large meals, alcohol intake, drugs, straining during micturition and defecation, and exposure to a warm environment. Other nonpharmacological strategies that are also recommended include elastic stockings and head-up tilt of the bed at night, increasing salt intake, and, in selected cases, cardiac pacing.117
A variety of pharmacological agents with different mechanisms of action have been used to reduce OH; fludrocortisone and desmopressin act through plasma volume expansion and by reducing natriuresis; clonidine and yohimbine both induce release of norepinephrine. Midodrine (adrenergic agonist activating alpha 1-receptors on arterioles and veins) increases peripheral resistance, thereby significantly reducing OH, as shown by three randomized, controlled trials.118–120 DL–Threo-dihydroxyphenylserine increases endogenous production of norepinephrine,121 whereas ergot derivatives are venoconstrictor agents with direct action on β2-receptors. Most specialists now consider fludrocortisone and midodrine as the first-choice drugs for this condition, the option between the two being made according to the individual characteristics of the patient. Controlled trials comparing the different symptomatic drugs used for OH are not available, and therefore the ultimate choice among them should be made according to the experience and judgment of the treating physician. Supine hypertension (SH) may occasionally be associated with severe OH. SH does not require drug treatment if systolic blood pressure is below 200 mm Hg; if treatment is required, short-acting calcium antagonists given at nighttime are commonly used.
Urinary symptoms in MSA are due to a complex mixture of central and peripheral nervous problems, sometimes superimposed on local pathology such as prostatic hypertrophy and perineal laxity.122 Peripherally acting anticholinergic drugs may help incontinence, but often at the expense of inducing retention; the administration of desmopressin at night may reverse nocturia. Intermittent self-catheterization or even an indwelling catheter may be needed in the presence of incomplete bladder emptying.
Male impotence can be partially circumvented by the use of intracavernosal papaverine, prostaglandin E1 or penile implants. A double-blind, placebo-controlled study showed that sildenafil is efficacious in the treatment of erectile dysfunction in parkinsonism due to MSA, but it may unmask or exacerbate orthostatic hypotension.123 Therefore, measurement of lying and standing blood pressure before prescribing sildenafil to men with parkinsonism is recommended.
Inspiratory stridor develops in about 30% of patients, possibly due to progressive degeneration of the nucleus ambiguus124 and consequent bilateral laryngeal abductor weakness. Continuous positive airway pressure may be helpful in some of these patients but in about 4% a tracheostomy is needed, after having considered all the ethical issues related to this procedure.125,126 Sleep apnea may also occur and should be managed appropriately.
PROGRESSIVE SUPRANUCLEAR PALSY
Progressive supranuclear palsy (PSP) is a chronic progressive neurodegenerative disorder characterized by continuation of clinical features including akinetorigid parkinsonism, postural instability supranuclear vertical gaze palsy, axial dyskinesia, and frontolimbic dementia.127–129 It was originally delineated as a novel heterogeneous system disorder by Richardson and colleagues130 and later termed PSP by Steele, Richardson, and Olszewski in their seminal report of 1964.131 Its unique neuropathology involves neurodegeneration with neuronal and oligodendroglial deposition of abnormally phosphorylated tau protein forming abundant neurofibrillary tangles (NFTs) in distinct basal ganglia and brainstem regions but also in the frontal cortex and dentate nucleus of the cerebellum.131–133 Subcortical NFT formation with granulovacuolar neuronal degeneration and gliosis are especially marked in the globus pallidus, subthalamic nucleus, substantia nigra, as well as in the midbrain and pontine reticular formation including the midbrain oculomotor complex and superior colliculi, periaqueductal gray, and basis pontis.
Abnormal intraneuronal deposits of tau protein are the pathological hallmark of PSP and a group of neurodegenerative disease that may either present with dementia or a parkinsonian movement disorder or combinations thereof and have accordingly been collectively grouped as “tauopathies” (Table 72-2). At the ultrastructural and molecular levels, however, there are differences between the types of tau aggregates and tau composition in these various disorders.134,135
TABLE 72-2 Neurodegenerative Diseases with Intraneuronal Accumulation of Abnormal Tau Protein (“Tauopathies”)
| Presenting with parkinsonism or other movement disorder | Progressive supranuclear palsy (PSP) |
| Corticobasal degeneration (CBD) | |
| Postencephalitic parkinsonism | |
| Guadeloupean parkinsonism | |
| Presenting with dementia | Alzheimer disease |
| Dementia pugilistica | |
| Down syndrome | |
| Pick disease | |
| Parkinsonism dementia syndromes | FTDP-17 |
| Parkinsonism-dementia-complex of | |
| Guam | |
| Niemann-Pick disease type C |
Epidemiology
There are no data on incidence and prevalence of PSP from population-based studies. Some hospital-based studies have found low prevalence rates of 1 to 2 cases per 100,000136,137 and an age-dependent rise in the incidence rate from 1.7 cases per 100,000 at age 50 to 59 to 14.7/100,000 at age above 80.137 Using a hospital linkage system of general practitioners in an area of London serving a population of more than 120,000, Schrag and colleagues138 arrived at an age-adjusted prevalence rate of 6.4/100,000, which is similar to the value of 5.0 calculated from a nationwide ascertainment study performed in the United Kingdom.139 Given the considerable rate of misdiagnosis and underdiagnosis of PSP shown in a number of clinical and neuropathological studies,139,140 it appears likely that the true prevalence is above these estimates.
Neuropathological series from specialized centers suggest that PSP may be the second most common type of degenerative parkinsonism after idiopathic Parkinson disease, accounting for around 6% of cases diagnosed with a parkinsonian syndrome in life.141
Clinical Presentation, Course, and Prognosis
The classic clinical syndrome of PSP is characterized by symmetrical rigid-bradykinetic parkinsonism with prominent extensor rigidity and dystonia of neck muscles producing an erect posture with neck hyperextension. Facial immobility with markedly reduced blink rates and elevated eyebrows with frontalis muscle overactivity cause a characteristic staring and astonished facial expression, and further clinical hallmarks include pronounced postural instability with recurrent falls, typically backward. Rest tremor is distinctly uncommon in PSP and most patients lack a meaningful response of their parkinsonism to trials of L-dopa. Abnormalities of eye movement are considered pathognomic and include slowing of vertical and horizontal saccades, hypometric saccades, and the development of a highly distinctive supranuclear vertical gaze palsy with complete loss of vertical eye movements and fully preserved excursions of vestibulo-ocular reflex movements.
The mean age at disease onset is around 60 years and mean survival is 6 years.142 The disease is progressive despite any therapy. In the late stage, PSP patients are wheelchair or bed bound. Their speech shows a characteristic growling and groaning. Dressing and feeding themselves is impossible. Marked difficulty in swallowing bears the danger of aspiration and consequent pneumonia.
Goetz and colleagues have studied the progression of global disability in PSP by defining median latencies from disease onset to certain milestones of key disabilities. The median delay to any key motor disability was as short as 48 months in their study of 50 patients with PSP; loss of ambulation, inability to stand, and a wheelchair-bound state were reached after a median of 5 years; speech had become unintelligible after a median of 6 years; and tube feeding was required after a mean of 87 months.143
In some instances it can be difficult to distinguish PSP from Parkinson disease patients during the first 2 to 3 years from symptom onset if the former do not yet clearly exhibit postural instability or ophthalmoplegia, and when they may still show a response to levodopa. Lees and coworkers140 presented evidence that the syndrome of PSP is probably heterogeneous. In a careful clinicopathological study of 103 consecutive cases of pathologically confirmed PSP, they assessed early clinical features occurring within the first 2 years after disease onset. Supranuclear gaze palsy was present in less than half of all cases, and falls only occurred in some 60% (Table 72-3). Taken together, about 50% of cases were characterized by early postural instability and falls associated with supranuclear vertical gaze palsy and cognitive dysfunction, corresponding to the classic pattern captured in current diagnostic criteria (Table 72-4). A second group of almost one third of their cases, however, had a parkinsonian phenotype with asymmetrical onset, rest tremor, and a moderate therapeutic response to levodopa, therefore providing greater room for diagnostic error regarding the differentiation from Parkinson disease. These authors propose to subdivide PSP into at least two clinically distinct subtypes termed “Richardson’s syndrome”, corresponding to the classic syndrome and “PSP-parkinsonism” with greater similarity to Parkinson disease (Table 72-5).
TABLE 72-3 Early Clinical Features (First 2 Years) in Progressive Supranuclear Palsy
| Clinical Feature | % |
|---|---|
| Falls | 60 |
| Impaired postural reflexes | 62 |
| Bradykinesia | 75 |
| Rigidity | 42 |
| Cognitive decline | 29 |
| Speech disturbance | 39 |
| Supranuclear gaze palsy | 38 |
| Abnormal pursuit or saccades | 44 |
| Nonspecific visual symptoms | 21 |
| Asymmetrical onset | 28 |
N = 103.
From Williams DR, de Silva R, Paviour DC, et al: Charcteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain 2005; 128:1247-1258.

TABLE 72-4 NINDS-SPSP Clinical Criteria for the Diagnosis of Progressive Supranuclear Palsy (PSP)
Rights were not granted to include this table in electronic media. Please refer to the printed book.
From Litvan I, Agid Y, Calne D, et al: Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996; 47:1-9.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree