Fig. 1
Neurolucida mapping of Hcrt cells in normal and narcolepsy. The cell counts are listed in each section. 3v third ventricle, Fx fornix, Mmb mammillary body, Opt optic tract
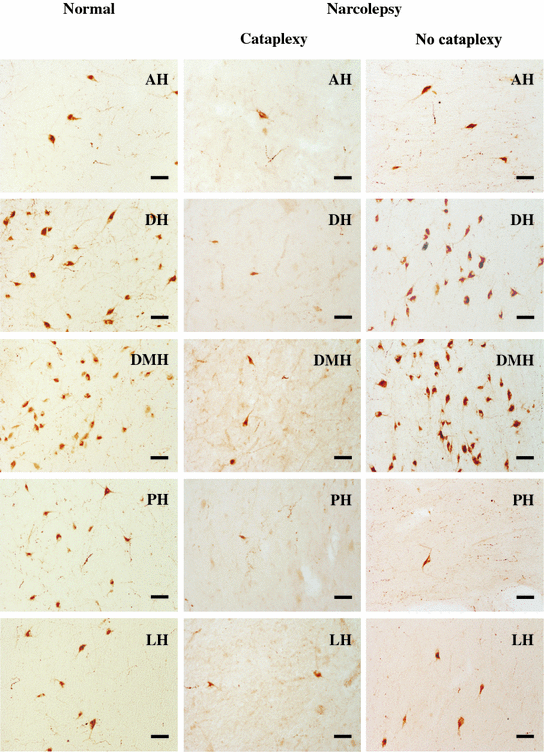
Fig. 2
Hcrt cells in the hypothalamic nuclei of normal and narcolepsy with and without cataplexy. AH anterior hypothalamus, DH dorsal hypothalamus, DMH dorsomedial hypothalamus, PH posterior hypothalamus, LH lateral hypothalamus, Scale bar–50 µm
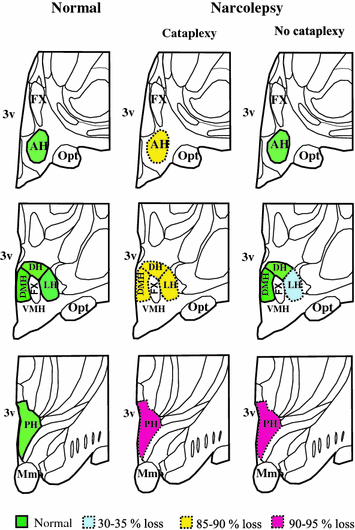
Fig. 3
Diagrammatic representation of the location of hcrt cells in the hypothalmus of normal brain and differential loss of Hcrt cells in narcolepsy with and without cataplexy. In normal, Hcrt cell somas are localized in AH, DH, DMH, PH and LH nuclei. In narcolepsy with cataplexy, cell loss was found in AH, DH, DMH, PH and LH nuclei, whereas, in narcolepsy without cataplexy cell loss was limited to LH and PH nuclei. AH anterior hypothalamus, DH dorsal hypothalamus, DMH dorsomedial hypothalamus, PH posterior hypothalamus, LH lateral hypothalamus, 3v third ventricle, Fx fornix, Mmb mammillary body, Opt optic tract, VMH ventromedial hypothalamus
The axon density reduction in Hcrt innervated nuclei in narcoleptics was positively correlated with the density of Hcrt axons in normal humans. The total number of Hcrt axons in all the structures analyzed was reduced by 67 % compared to an 89 % reduction of Hcrt soma count in the same brains, suggesting that Hcrt cells with smaller axonal fields are lost to a greater extent in narcolepsy or that axonal sprouting occures in surviving Hcrt cells (Thannickal et al. 2003). 99 % of Hcrt neurons are Narp positive (Reti et al. 2002).The number of Narp-positive neurons was reduced by 89 % in these areas of the narcoleptic hypothalamus (Blouin et al. 2005; Crocker et al. 2005).
4 Hypocretin Cell Loss in Narcolepsy Without Cataplexy
The narcolepsy without cataplexy patient whose complete brain was available for study had an overall loss of 33 % of Hcrt cells compared to normals, with maximal cell loss in the posterior hypothalamus. The maximum percentage reduction occurred in the posterior hypothalamic nucleus (95 % loss). There was no Hcrt cell loss in anterior, dorsal and dorsomedial nuclei of the narcolepsy without cataplexy patient (Figs. 1, 2 and 3). There was no reduction in the number of MCH neurons in either type of narcolepsy (Thannickal et al. 2009).
Work in animals (Gerashchenko et al. 2003) has shown that cerebrospinal Hcrt levels can be normal even when there is a substantial loss of Hcrt cells. CSF levels may be a function not only of the percentage of Hcrt cells lost, but also the activity and the mean distance of surviving Hcrt cells from the ventricles. Thus, patients with loss of posterior hypothalamic Hcrt cells may still have normal CSF levels of the peptide, even though it is not being synaptically delivered to the cells normally receiving hcrt axonal projections (Oka et al. 2006). Parkinson disease patients have a loss of Hcrt neurons, although to a lesser extent than in narcolepsy with cataplexy (Thannickal et al. 2007; Fronczek et al. 2007). These patients have many of the symptoms of narcolepsy; however, distinct episodes of cataplexy have not been reported. This is consistent with the current observations of narcolepsy without cataplexy with partial loss of Hcrt neurons.
4.1 Elevated Glial Fibrillary Acidic Protein Levels in Narcolepsy
Elevated Glial fibrillary acidic protein (GFAP) levels is an established indicator of astrogliosis. This process is characterized by rapid synthesis of GFAP and is demonstrated by an increase in protein content (Eng et al. 2000). GFAP levels in the CSF of narcoleptics (Feneberg et al. 2013) may represent hypothalamic gliosis and support the hypothesis of a neurodegenerative process (Thannickal et al. 2000). There was a significant increase in gliosis indicated by GFAP staining and a significant difference in the amount of gliosis across hypothalamic nuclei in narcoleptics (Fig. 4). Narcoleptics with cataplexy had increased GFAP staining throughout the hypothalamus, with a maximum percentage GFAP increase in the posterior hypothalamic nucleus. The number of Hcrt axons in the anterior hypothalamus was normal in narcolepsy without cataplexy (Thannickal et al. 2009). We speculate that high GFAP levels may be a differential biomarker in some sleep diseases such as secondary hypersomnia in Prader–Willi syndrome or neurologic disorders such as in certain cases of Guillain–Barré syndrome, both of which also may present with low CSF hcrt-1 levels (Mignot et al. 2002). In conclusion, GFAP may be useful as an additional disease biomarker in patients with narcolepsy.
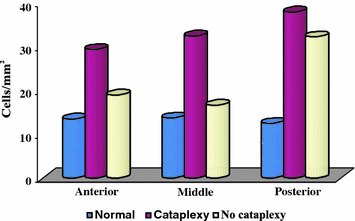

Fig. 4
Gliosis in narcolepsy. Glial fibrillary acidic protein labeled astrocytes (GFAP) density (cells/mm2) in normal and narcoleptic brains of the hypothalamus
4.2 Changes in Hypocretin Neuronal Expression with Normal Aging in the Human Hypothalamus
It has been found that Hcrt concentration in the cerebral spinal fluid (CSF) of infants increased from birth until 2–4 postnatal months and then decreases throughout childhood and puberty (Feneberg et al. 2013). However, Aran et al. (2012) found no change in CSF Hcrt concentration between birth and 4 years of age. In older humans, the number of Hcrt neurons was unchanged between 50 and 90 of age (Kanbayashi et al. 2002; Fronczek et al. 2005). There was 24 % decrease in the number of Hcrt neurons in adults compared with infants and children (Fronczek et al. 2012). This may contribute to changes in sleep regulation during development and with aging. Animal studies have shown that decreased Hcrt expression is correlated with changes in sleep regulation with aging (Hunt et al. 2015; Brownell and Conti 2010).
4.3 Molecules Co-expressed in Hypocretin Neurons
Hcrt deficiency is directly involved in several neurological disorders. Dynorphin, glutamate and secretogranin II are found co-localized within Hcrt cells (Sawai et al. 2010; Chou et al. 2001; Bayer et al. 2002). There are several receptors and transporters that are expressed in Hcrt cells such as 5HT1-A, adenosine A1-R, GABA A alpha 3, GABA A epsilon, GABA B, group III metabotropic glutamate receptors, leptin R, Y4-R, vGlut1 and vGlut2 (Torrealba et al. 2003; Collin et al. 2002; Thakkar et al. 2002; Bäckberg et al. 2003, 2004; Moragues et al. 2003; Acuna-Goycolea et al. 2004; Håkansson et al. 1999; Rosin et al. 2003; Campbell et al. 2003). Acetylcholinesterase E, STAT-3, Narp and neuroglobin (Chou et al. 2004; Reti et al. 2002; Håkansson et al. 1999; Hundahl et al. 2008) are also found in Hcrt cells. Hcrt cell loss also causes the deficiency of these molecules. Their role in the symptoms of narcolepsy is not known.
5 Conclusions
The identification of hypocretin/orexin deficiency as the cause of human narcolepsy and the potential role of hypocretin peptides in other neurological disorders has sparked interest in the pathophysiology of the Hcrt system. As narcolepsy is found in about 1 of 2000 humans, it is the third most prevalent type of neurodegenerative disease, behind Alzheimer’s (the most common) and Parkinson’s (which affects about 1 in 1000 humans) (Dorsey et al. 2005), but more prevalent than Huntington’s or amyotropic lateral sclerosis (each about 1 in 5000).
Acknowledgements
Tissue specimens were obtained from the Human Brain and Spinal Fluid Resource Center, West Los Angeles Healthcare Center, Los Angeles, CA. This study was supported by the Medical Research Service of the Department of Veterans Affairs and NIH grants NS14610 and MH064109 to J.M.S.
References
Acuna-Goycolea C, Li Y, Van Den Pol AN (2004) Group III metabotropic glutamate receptors maintain tonic inhibition of excitatory synaptic input to hypocretin/orexin neurons. J Neurosci 24:3013–3022CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







