Pathophysiology of Myoclonic Epilepsies
Renzo Guerrini*
Paolo Bonanni*
Lucio Parmeggiani*
Mark Hallett†
Hirokazu Oguni‡
*Epilepsy, Neurophysiology, Neurogenetics Unit, University of Pisa and Research Institute ‘Stella Maris’ Foundation, Pisa, Italy
†Human Motor Control Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland
‡Department of Pediatrics, Tokyo Women’s Medical University, Tokyo, Japan
Introduction
Relationship between Myoclonus and Epilepsy
Myoclonus can be defined as an involuntary movement, which is brief and jerky and involves antagonist muscles. It can either originate from abnormal muscle activation in the form of brief electromyographic (EMG) bursts (positive myoclonus) or, more rarely, from brief interruptions of ongoing electromyographic activity (negative myoclonus).
Myoclonus and its association with epilepsy was first described by Dubini (1) in 1846. He described a cohort of patients with possibly different conditions who had as a common feature involuntary jerky movements that he named “electric chorea.” The first attempt at classification of myoclonus came much later, when Lundborg (1903) (2) recognized three etiological categories: (a) symptomatic myoclonus, (b) essential myoclonus, and (c) familial myoclonic epilepsy (subdivided into nonprogressive and progressive forms). Later, Muskens (1928) (3) highlighted a close nosologic link between myoclonus and epilepsy and coined the term “fragments of epilepsy” to designate the myoclonic jerks of patients with epilepsy. Since then, many other conditions in which myoclonus is a significant symptom have been reported, allowing the following etiological classification of myoclonus (4,5): (a) physiologic myoclonus (sleep related, hiccup, and myoclonus induced by anxiety or exercise), (b) essential myoclonus (subjects without other neurologic signs), (c) epileptic myoclonus (conditions in which the predominant element is epilepsy), and (d) symptomatic myoclonus (conditions in which the predominant element is encephalopathy). According to Marsden et al. (4) and Fahn et al. (5), the category of epileptic myoclonus comprises “fragments of epilepsy” and includes forms that originate from an isolated spike discharge in the motor cortex. Concepts on nosology of myoclonic epilepsies have evolved considerably in recent years (6,7), but the use of the term “epileptic myoclonus” is still confusing. Some authors define epileptic myoclonus as that which occurs within the setting of epilepsy and has the epileptic spike as the neurophysiologic hallmark (8). Others define epileptic myoclonus as those forms in which a paroxysmal depolarization shift is thought to be the underlying neurophysiologic substrate, irrespective of which population of neurons (cortical or subcortical) is primarily involved (9) and of the possibility of obtaining a time-locked electroencephalographic (EEG) correlate. Frequently, however, the EEG correlate of myoclonus can only be detected by using jerk-locked [EEG or magneto encephalographic (MEG)] averaging or coherence analysis. Considering the limitations related to these technical difficulties, it has recently been suggested that epileptic myoclonus could be comprehensively defined as an elementary electroclinical manifestation of epilepsy involving descending neurons, whose spatial (spread) or temporal (self-sustained repetition) amplification can trigger overt epileptic activity (9).
Epileptic Myoclonus
Epileptic myoclonus includes positive and negative cortical myoclonus, thalamocortical myoclonus, and reticular myoclonus (Fig. 3-1) (10).
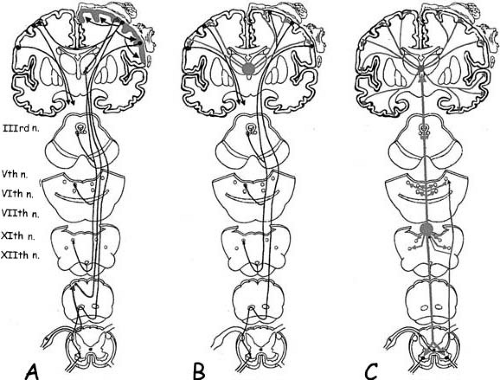 FIG. 3-1. Schematic drawing showing three main neurophysiological mechanisms of myoclonus. A: Cortical myoclonus. Myoclonic-related cortical activity emanates directly or via reflex sensory pathway activation (upward arrow), from a hyperexcitable sensorimotor cortex (gray shading). A descending volley then activates different muscles through brainstem and spinal motor neurons, according to a rostrocaudal pattern. Myoclonic-related cortical activity can intracortically spread within the same hemisphere or to the contralateral hemisphere through the corpus callosum (black arrows). B: Idiopathic (primary) generalized epileptic myoclonus. Myoclonic-related cortical activity originates subsequent to afferent volleys from subcortical structures (gray shading) that act synchronously on a diffusely hyperexcitable cortex. As a consequence, muscles from both sides are activated synchronously and muscles innervated by the cranial nerves are involved through a rostrocaudal pattern of activation, as in cortical myoclonus. This suggests that a descending volley passes through the brainstem. However, it is also possible for the descending volley to pass through a polysynaptic, thalamo-reticular pathway (see text, Thalamo-Cortical myoclonus—Neurophysiologic Findings). C: Reticular reflex myoclonus. Myoclonic-related activity originates from the reticular formation in the medulla (gray dot and upward gray arrows). Surface EMG recordings show an initial activation of the trapezius muscle (XIth cranial nerve), followed by the sternocleidomastoid (XIth cranial nerve), then the orbicularis oris (VIIth cranial nerve), and finally the masseter (Vth cranial nerve). EEG recordings show diffuse abnormalities, widely projected over both hemispheres, which are not time locked to the myoclonic EMG burst and often follow muscle activation. This supports the fact that they are not related to myoclonus generation. |
Clinically, epileptic myoclonus may be positive or negative. It is focal if it involves a restricted, usually distal, group of muscles; multifocal, when asynchronous focal jerks involve different body areas; or generalized, when jerks involve most body segments in an apparently synchronous manner. Furthermore, it may be spontaneous; or reflex, if induced by movement or by sensory or visual stimuli. Finally, as regards periodicity, epileptic myoclonus may be rhythmic or arrhythmic.
The neurophysiological characteristics of epileptic myoclonus (8,9,10) are (a) duration of the myoclonic electromyography (EMG) burst ranging between 10 and 100 msec; duration of the EMG silent period of negative myoclonus, ranging from 50 to 400 msec; (b) synchronous EMG bursts or silent periods on antagonist muscles; (c) presence of an EEG correlate detectable by routine surface EEG or burst-locked EEG averaging. The EEG correlate is time locked in cortical reflex myoclonus and in primary generalized epileptic myoclonus. Secondarily generalized epileptic myoclonus, as defined in this chapter, has a time-locked EEG correlate, but the temporal relationships between EEG and EMG event may vary according to the pattern of muscle spread. Reticular reflex myoclonus may have an EEG correlate, which, however, is not time locked.
Cortical Myoclonus
Clinical Findings
Cortical myoclonus can be positive or negative; focal, multifocal, or generalized; spontaneous or reflex; and rhythmic or arrhythmic.
Neurophysiologic Findings
Origin
Cortical myoclonus originates from abnormal neuronal discharges in the sensorimotor cortex. Abnormally firing motoneurons may be primarily hyperexcitable or may be driven by abnormal inputs originating from hyperexcitable parietal (11) or occipital (12) neurons. Each jerk represents the discharge from a small group of cortical motoneurons somatotopically connected to a group of contiguous muscles. A cortical potential that is temporally and consistently correlated with the myoclonic potential, and localized on the contralateral sensorimotor region, can be demonstrated by EEG, MEG, or jerk-locked averaging (JLA) (13,14,15,16). In some patients with focal myoclonus, epilepsia partialis continua, and focal motor seizures, excision of a small cortical region, identified by electrophysiological recordings as the area of origin of the myoclonic discharges, leads to remission of symptoms (17,18,19). However, in epilepsia partialis continua, the cortical site of origin of the myoclonic discharges and the site of origin of the associated focal seizures do not necessarily coincide (7).
In patients with progressive myoclonus and epilepsy (PMEs), the polarity of the premyoclonic potential as detected by JLA is positive (14,15,20,21,22,23,24,25,26). In contrast, in patients with Alzheimer’s disease, Down syndrome, and in some patients with Lennox–Gastaut syndrome LGS, there is a negative sharp wave associated with myoclonus (27,28,29). Mima and co-workers (16) used MEG and EEG to study patients with cortical myoclonus caused by PMEs, familial cortical myoclonic tremor (see specific section, “Rhythmic or Arrhythmic Recurrence” of cortical myoclonus), corticobasal degeneration, Alzheimer’s disease, and LGS. In all patients, jerk-locked MEG averaging revealed cortical activities associated with myoclonic jerks. The estimated generator of the earliest peak of the premyoclonus cortical activity was localized at the contralateral precentral gyrus. As judged from the direction of the electric current, surface-positive activity was detected in PMEs, familial cortical myoclonic tremor, and corticobasal degeneration; and negative activity, in Alzheimer’s disease and LGS. According to Mima and colleagues (16), the negative premyoclonic potential may be generated by a hyperexcitable motor cortex, through a mechanism of epileptogenesis [i.e., in the motor cortex, the paroxysmal depolarization shift (PDS) is widely distributed]. The positive polarity potential is thought instead to be generated by a PDS restricted to the deep layers (probably in lamina V) of the motor cortex (16,22). However, there is good evidence from other authors (18,19) that cortical hyperexcitability may reside in sensory or parietal regions rather than in the motor cortex. If this were the case, then it is conceivable that premyoclonic potentials of opposite polarity could occur simply because of the reversal in anatomic orientation of pyramidal cells in motor and sensory parts of the central sulcus. Jerk-locked MEG is more sensitive than jerk-locked EEG averaging, at least in some patients, in detecting cortical activity associated with myoclonus (22), possibly because the magnetic fields are not attenuated by the skull.
Induction Mechanisms
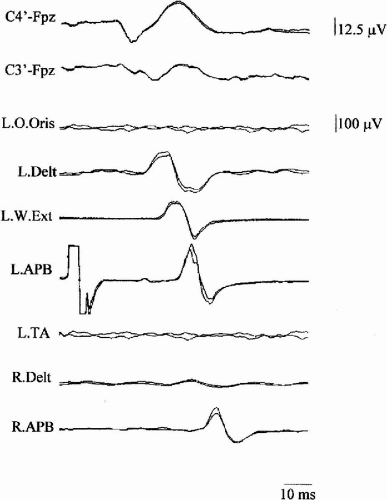
In patients with cortical reflex myoclonus, appropriate stimuli administered to a resting somatic segment produce a reflex muscle response (jerk), which, in normal subjects, can only be detected during voluntary contraction. The normal reflex pattern from mixed nerve stimulation consists of an H response after ~28 msec and a long latency reflex (LLR II) at ~50 msec. An earlier reflex component at ~42 msec (LLR I) is present in 30% of normal subjects, and at ~70 msec an additional LLR III may occur (30). The reflex jerk was originally called a C- (cortical) reflex, as it was presumed to be cortically mediated (31). Electric or mechanic stimulation of a relevant nerve produces a reflex response that has a latency of 30 to 50 msec for the upper limb and 60 to 70 msec for the lower limb. If the afferent [N20 of somatosensory evoked potentials (SEPs)] and efferent motor-evoked potentials (MEPs) from transcranial magnetic stimulation (TMS) conduction times are subtracted from these latencies, the cortical relay time (CRT) is obtained, corresponding to the intracortical transmission time of myoclonic activity. SEPs of giant amplitude are often observed in patients with cortical reflex myoclonus (CRM) (Fig. 3-2). The giant components are most often P25/P30 (P1) and N35 (N2) (32), and their generators are localized close to the central sulcus (26,33). Because the subcortical components and the first cortical component (N20) have normal amplitude, an abnormality of intracortical inhibition following arrival of the first volley of thalamocortical activity could be the cause both of the abnormal enlargement of the giant SEP components and of activation of descending motor outputs leading to the C-reflex (32). The striking resemblance in latency and morphology of the giant SEPs to the myoclonus-related cortical spike suggests that both originate from common cortical mechanisms (15).
In the typical forms of CRM, the reflex jerk in the hand has a latency of ~50 msec and the CRT has a mean duration of 7 msec (34). Typical CRM can be observed in patients with focal cortical lesions (31), spinocerebellar degeneration (14,22,35), multiple system atrophy (22,36,37), cerebral anoxia (14,38), childhood metabolic degenerations, such as neuronal ceroid lipofuscinosis and sialidosis (30,33), Alzheimer’s disease, Down syndrome (27,28), and mitochondrial disorders (39,40,41).
If the latency of the reflex myoclonus is reduced to ~40 msec, that is to say ~10 msec shorter than usual and ~2 msec longer than the sum of the afferent and efferent times to and from the cortex, CRM is defined as atypical. Atypical forms have been observed in patients with epilepsia partialis continua (21,42), postanoxic myoclonus (35), PMEs (30,33), neuronal ceroid lipofuscinosis (32), Huntington disease (42), and corticobasal degeneration (33).
It has been hypothesized that the different patterns of abnormality in CRM might be explained by differences in the processing and relay of sensory information in thalamocortical pathways (30,34). In the typical forms, CRM may involve abnormal relays through the sensory cortex to the motor cortex, either directly or via cerebellar–thalamocortical projections (32,34). In the atypical forms, myoclonus may represent enhancement of a direct sensory input to the motor cortex (34).
A form of CRM characterized by more prolonged C-reflex latency has been described in Rett syndrome (44). Clinically, myoclonus is multifocal, predominating distally, and arrhythmic. A positive potential, localized on the contralateral centroparietal area, precedes myoclonus with a latency of 34 ± 7 msec for the forearm muscle. This is compatible with corticomotoneuronal conduction. The N20–P30 and P30–N35 components of the SEPs have significantly increased amplitude. In addition, the latency of the N20 component is delayed and the N20–P30–N35 interval is significantly increased and has expanded morphology. The latency between electric stimulation (median nerve) and onset of reflex myoclonus is 65 ± 5 msec when the recording is made from the abductor pollicis brevis (APB) muscle. Topographic mapping of the SEP voltage shows, for the P30 component, a field distribution very similar to that of the premyoclonic potential. The CRT has a duration of 28 ± 4 msec— a value that is three- to four-fold higher than that observed in PMEs (34). It is, therefore, probable that in Rett syndrome the following sequence of events occurs: slight delay in central conduction of the impulse afferent to the sensorimotor cortex (N20), slowing of the processing of the afferent impulse (interval N20–P30; mean = 11 msec), delay in corticocortical transmission to the precentral neurons subserving movement of the stimulated body segment (latency increase P30-C-reflex; mean = 32 msec), and rapid descending volley to the spinal motoneurons. Thus, the premyoclonic EEG and the corresponding P30 wave would represent a discharge arising from the postcentral neurons, subsequently slowly activating the motor efferences through connections with the precentral neurons (45). Intracortical conduction time could be particularly prolonged due to the synaptic abnormalities present in the brain of Rett patients with mutation in MECP2 gene (46).
One particular form of CRM may be induced by photic stimuli, in idiopathic generalized epilepsies (benign myoclonic epilepsy, juvenile myoclonic epilepsy), in idiopathic generalized photosensitive epilepsies, or in some forms of cryptogenic epilepsies (myoclonic-astatic epilepsy and severe myoclonic epilepsy). The most active frequency of stimulation is between 10 and 20 Hz. The ratio between stimulus (flash) and reflex response (jerk) is not constant and is only partly time locked. Responses are usually symmetric and predominate in the upper limbs. In most cases, they are mild, only producing head-nodding and slight arm abduction. More generalized jerks, involving the face, trunk, and legs, may occasionally cause the patient to fall. Isolated myoclonic jerks occur without loss of consciousness. However, generalized jerks may be repeated, especially if the stimulus is protracted. In this situation consciousness may be impaired and a generalized tonic–clonic seizure may follow. The relationship of myoclonic jerks to the stimulus is complex. Sometimes there is no definite time relationship. On other occasions, the jerks may be repeated rhythmically with the same frequency as the stimulus or at one of its subharmonics (47). If the triggering stimulus is prolonged, the clinical response may translate into a generalized convulsion (48).
In patients with diffuse degenerative brain damage (49,50) or with occipital lesions (11), when intermittent photic stimulation (IPS) is performed at low frequencies (0.5–3 Hz), each flash may provoke a frontal giant potential, which is time locked to the stimulus and precedes by ~15-msec myoclonic jerk localized in the face or spreading from the face in a rostrocaudal pattern. Further repetition of the stimulus may induce a generalized tonic–clonic seizure (50). The potential evoked on the occipital cortex may be normal or giant. Because the occipital response precedes the giant frontocentral response by ~4 msec, which may correspond to the time required for the impulse to pass from the occipital to the frontal cortex, long pathways of occipitofrontal intracortical transfer have been hypothesized (51). Study of the recovery cycles of the EEG components in response to a pair of flash stimuli has shown that components on the central regions recover more rapidly than those on the occipital regions. Brain-mapping analysis indicates that the frontal activity correlated with the myoclonus originates in the premotor and motor cortices. Therefore, hyperexcitability of the visual cortex was not considered an essential prerequisite in this type of myoclonus by some authors (49,50).
The orbitofrontal photomyoclonic response, which is widely described in the EEG literature, in adults undergoing IPS (synonyms: frontopolar response, recruiting response, photooculoclonic response), is probably a form of photic CRM which may also be observed in normal individuals. The frequency of flashes that is effective in triggering this response is usually between 8 and 20 Hz. Patients present with rapid myoclonic jerking of the periorbital muscles producing fluttering of the eyelids and blinking, which is synchronous with the flashes. There may be vertical oscillations of the eyeballs. Amplitude of the response increases progressively during the first flashes, reaching a maximum within a few seconds. The maximal amount of muscle activity is initially observed in the inferior orbicularis oculi muscles with subsequent irradiation to other facial muscles, the frontal and occipital areas, and the neck (52). Further spread may be seen if IPS stimulation is protracted. The response is blocked by opening of the eyes or cessation of IPS. Although the pathophysiology and significance of the orbitofrontal photomyoclonic response have long been disputed, our current understanding indicates it to be an expression of cortical origin (53) within the spectrum of photic CRM (50).
Photic CRM provides a clear example of how a single jerk (fragment of epilepsy) can gradually translate into overt seizure activity, through a temporal summation effect of the triggering stimuli.
Rhythmic or Arrhythmic Recurrence
A focal spike generated in the sensorimotor cortex can produce a focal myoclonic jerk. Rhythmic or arrhythmic jerk recurrence may lead to epilepsia partialis continua (4). The cortical origin of myoclonus in epilepsia partialis continua has been widely demonstrated (18,19,22,54,55,56). However, a subcortical origin has also been proposed, at least in some patients showing basal ganglia or cerebellar lesions and no jerk-locked EEG activity (56,57,58,59,60).
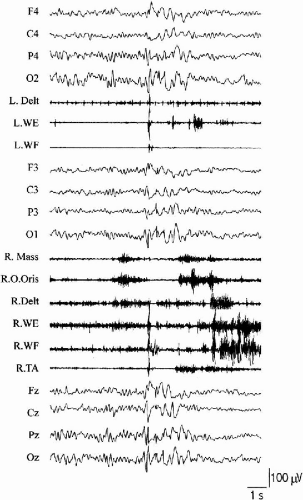
Bilateral, rhythmic, virtually continuous myoclonus at 11 to 18 Hz is typically observed in Angelman syndrome (61). The jerks are spontaneous at rest and, if particularly intense, may produce dystonic posturing of the upper limbs or the feet. A cortical transient in the contralateral sensorimotor cortex precedes each EMG burst by an interval consistent with rapid corticomotoneuronal conduction (20–30 msec). Clinical and neurophysiological characteristics suggest a high propensity for intrahemispheric and interhemispheric cortical spread of myoclonic activity that can be manifested as apparently generalized myoclonic jerks (Fig. 3-3). There is no giant SEP and lack of C-reflex hyperexcitability correlates with the absence of reflex jerks. The post-MEP silent period has short duration and testifies to a deficit of inhibitory cortical mechanisms (62). The pattern of myoclonus observed in Angelman syndrome suggests that small areas within the motor cortex are able to independently produce hypersynchronous, rhythmic neuronal discharges recruiting muscle activity similar to tremor. Distal myoclonic jerks can convert into overt generalized myoclonic status (61). As this pattern of myoclonus is observed in all patients with Angelman syndrome, irrespective of their genetic class, mutations in the UB3A gene must play a direct role in its genesis. Transition to overt seizure activity in Angelman syndrome may in turn be facilitated by reduced representation of GABAA subunit receptors, as demonstrated by the much more frequent and severe epilepsy observed in patients bearing a chromosome 15q11-13 deletion, leading to reduction of gene product.
A rhythmic pattern of cortical myoclonus, bearing some similarities to that seen in Angelman syndrome, may be observed in association with different clinical conditions. Schulze–Bonhage and Ferbert (63) described a patient who developed cortical action tremor and focal motor seizures of the left hand, following a right parietal infarction. Wang and co-workers (64) reported cortical tremor appearing after surgical removal of a frontal lobe meningioma.
A similar rhythmic pattern was produced as a reflex response to tapping on the forehead or by sudden acoustic stimuli in some patients with Down syndrome (65) and tuberous sclerosis (9). Cortical tremor, in the form of postural or action tremor, and showing the neurophysiological characteristics of reflex cortical myoclonus, can also be observed in patients with PMEs (24,25) or with familial adult myoclonic epilepsy (FAME) (66,67,68) and autosomal dominant cortical reflex myoclonus and epilepsy (ADCME) (see Chapter 21).
In rhythmic cortical myoclonus, EEG–EMG frequency analysis (69,70) proved superior to jerk-locked backaveraging in detecting cortical correlates of rhythmic myoclonic jerks. Repetitive myoclonic jerks can produce a sort of rhythmic, almost synousoidal EEG correlate on backaveraged data, with the maximum amplitude coincident to the applied trigger point (Fig. 3-4). In this situation, the definition of the proper EEG peak from which latency to EMG bursts can be measured becomes arbitrary. Therefore, application of frequency analysis by means of discrete Fourier transform to both EEG and EMG signals appears appropriate to overcome the methodological limitation of backaveraging. The linear association of different rhythmic signals can then be estimated using coherence analysis. Coherence measures the linear association between two signals and can take values from 0 to 1, where 0 indicates no linear association and 1 indicates a perfect linear association. Coherence between scalp EEG and surface EMG activity has been used to detect functional coupling between oscillatory activity in the motor cortex and that in muscle in physiologic and pathologic conditions (71,72). Grosse and co-workers (70) applied EEG–EMG and EMG–EMG coherence analysis to the study of rhythmic cortical myoclonus (ADCME, Angelman syndrome, LGS, and celiac disease) and compared results with those generated by traditional backaveraging. Coherence analysis proved more sensitive than traditional backaveraging and showed an exaggerated coherence between EEG and contralateral EMG and between pairs of ipsilateral, distal EMG signals in the band that corresponded to the more represented frequencies on EMG channels. These findings support a cortical driving for rhythmic EMG activity (70).
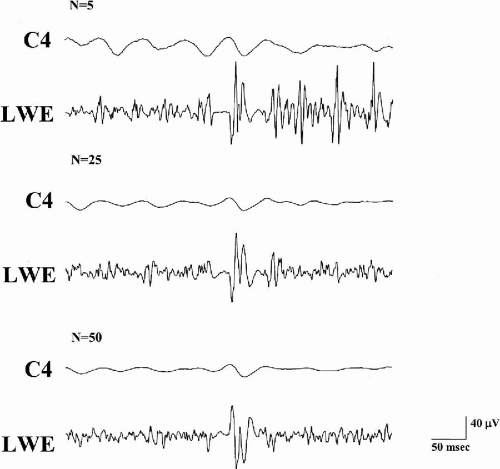 Fig. 3-4. EEG activity is backaveraged using the onset of myoclonic jerks in left wrist extensor (LWE) muscle as a trigger in a patient with Angelman syndrome. Letter N indicates the number of replications. A positive–negative–positive transient gradually emerges from background noise after increasing the number of averaged EEG epochs. However, other EEG transients are observed and appear time locked to other EMG bursts. This is due to the repetitive, rhythmical appearance of cortical myoclonus in Angelman syndrome. A different approach is needed to better assess the correlation between EEG and EMG activity.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|