Pediatric Arteriovenous Malformations
Vascular malformations of the central nervous system are a heterogeneous group of lesions that occur in both the brain and the spinal cord. Like those in other anatomical sites, vascular anomalies of the central nervous system can be classified according to rheologic characteristics (fast-flow and slow-flow) and by channel composition (i.e., arteriovenous malformation [AVM], cavernous malformation [CM], and venous malformation [VM]) and may occur either independently or in association with syndromic conditions. AVM is arguably the most important vascular anomaly in the nervous system in children; it is relatively common and usually requires treatment given the risk for hemorrhage.
67.1 Pathophysiology
AVMs consist of direct arterial-to-venous connections without intervening capillaries; they occur in the cerebral hemispheres, brainstem, and spinal cord. Functional neural tissue does not reside within the lesion.1 Anomalies range from simple arteriovenous fistulas to complex tangled channels connecting enlarged feeding arteries to draining veins.
AVMs may increase in size over time or may demonstrate changes in the caliber of component vessels, which may portend a risk for hemorrhage. These changes may occur from “mechanical” dilation as the result of increased flow through poorly differentiated vessels and the recruitment of collateral arterial feeders. Ischemia and surrounding microhemorrhages with resultant gliosis may promote the enlargement of an AVM by destruction of the surrounding parenchyma. Expansion of an AVM may also result from the growth of new blood vessels (i.e., angiogenesis, which is a complex process regulated by a wide range of proteins, including metalloproteinases and related growth factors, such as vascular endothelial growth factor).2–8 Enlargement of an AVM seems to alter the adjacent brain. Functional magnetic resonance (MR) imaging data have suggested that the presence of an AVM in an eloquent area of the brain may be associated with “migration” of the function to adjoining cortex or to the homologue on the contralateral hemisphere.9,10 This migration or displacement of functional neuronal tissue has important implications for surgical planning.
67.2 Epidemiology
AVM is the most common symptomatic high-flow intracranial vascular abnormality in adults and children.11 In a large autopsy series, the overall frequency of detection for AVMs was 1.4% (46 of 3,200 cases of brain tumor).12 In another report, the annual incidence of symptomatic AVMs was 1.1 per 100,000.13 The pediatric age group accounts for 12 to 18% of all AVMs from major centers, and the overall prevalence in children is about 0.02%.14–17 Most AVMs present in adulthood, with a mean age of patients at presentation of approximately 30 to 40 years. About 20% of all symptomatic AVMs present before 15 years of age.18 There is no sex predilection for pediatric AVMs. A number of AVMs are associated with underlying genetic conditions. The RASA1 mutation, resulting in familial AVMs and/or cutaneous capillary malformations, has been associated with symptomatic cerebral AVMs in a small number of families.19 Hereditary hemorrhagic telangectasia is a genetic condition that predisposes affected individuals to AVMs. Thirty-five percent of pediatric cases were associated with hereditary hemorrhagic telangectasia. Twenty-three percent of patients had multiple AVMs, with a mean age at presentation of 35 years13
67.3 Presentation
The neurologic signs and symptoms of central nervous system vascular anomalies correlate with the anatomical site of involvement and the age at presentation. The lesions can be deep in the parenchyma, superficial, or located in the dural and arachnoid coverings. In general, AVMs that are symptomatic at or soon after birth are extremely fast-flow lesions and often are associated with high-output cardiac failure. The more common pial AVMs usually present with hemorrhage later in childhood or adulthood. The likelihood and severity of symptoms and the probability of hemorrhage caused by the vascular anomaly depend on multiple factors, including type of malformation, size, location, and hemodynamic and angioarchitectural characteristics.
Hemorrhage and seizure are the most common presenting symptoms of pediatric AVM, but the findings may also include headache, focal neurologic deficits, and cognitive decline.20–23 A substantially large number may be asymptomatic.
Children with AVM are more likely than adults to present with intracranial hemorrhage: 80 to 85% in some pediatric series.15,24 For children known to have an AVM, the annual risk for hemorrhage has been estimated at 2 to 4%.25,26 Hemorrhagic events from an AVM in childhood have been associated with a 25% mortality rate.27 In contrast to earlier reports suggesting that smaller AVMs may be associated with a higher risk for bleeding, more recent data have shown that size is not a major determinant in the risk for hemorrhage.28,29
The hemorrhage can produce seizures, headache, or focal neurologic deficits. The bleeding associated with an AVM is most often an intraparenchymal hemorrhage, although subarachnoid hemorrhage and intraventricular hemorrhage are also common. Bleeding can occur in any one location or in all three sites together.30–32 A nontraumatic intraparenchymal hemorrhage in a child should raise concerns for the presence of an AVM or tumor. Rates of rebleeding have been reported to be approximately 6% within the first 6 months.
Independently of hemorrhage, AVMs can produce deficits from mass effect or from cerebral ischemia that is due to the diversion of blood to the AVM from the normal cerebral circulation (“steal”). The presentation of symptoms in AVM is generally acute if related to hemorrhage or seizure (often occurring within minutes to hours) but is chronic (over months) if related to a steal phenomenon or headache.
67.4 Physical Signs
Systolic bruits over the eye or through the head and or fontanels are suggestive of an AVM; focal neurologic signs help localize the lesion. A bruit is found in 15 to 40% of patients with AVM. It is especially common if branches of the external carotid arteries are involved and best heard over the ipsilateral eye or mastoid region. Large, pulsatile vessels may be present in the scalp, face, and neck, and vascular anomalies can be found in retina. Major arteriovenous shunting may also be associated with tachycardia, cardiomegaly, and even cardiac overload, especially in infants and children and particularly when the vein of Galen is involved. Other than potential neurologic deficits, the typical patient will not have any obvious findings on general physical examination to suggest an underlying AVM.
67.5 Imaging
The comprehensive evaluation of a patient with an AVM includes a detailed history, neurologic and physical examination, and radiographic studies to delineate the anatomy of the lesion.33 The vast majority of patients present with the new onset of a neurologic deficit, such as an unusually severe headache (“worst headache of my life”) or seizure due to acute hemorrhage. These patients should be screened with computed tomography (CT) (▶ Fig. 67.1, ▶ Fig. 67.2). CT is a reliable indicator of recent intracranial hemorrhage, may permit localization of the source of bleeding, and facilitates the early diagnosis of AVM. Because of its speed, availability, and ease of use, CT angiography has increasingly been employed as an initial study upon presentation to the emergency department.34 CT angiography better delineates AVM, particularly in the setting of an acute hemorrhage. In addition, the index of suspicion for an AVM is high whenever an intraparenchymal hemorrhage is found in a child or young adult without an antecedent history of trauma.35 Repeated imaging in 4 to 6 weeks is indicated to evaluate the hemorrhagic cavity after the clot has cleared.36
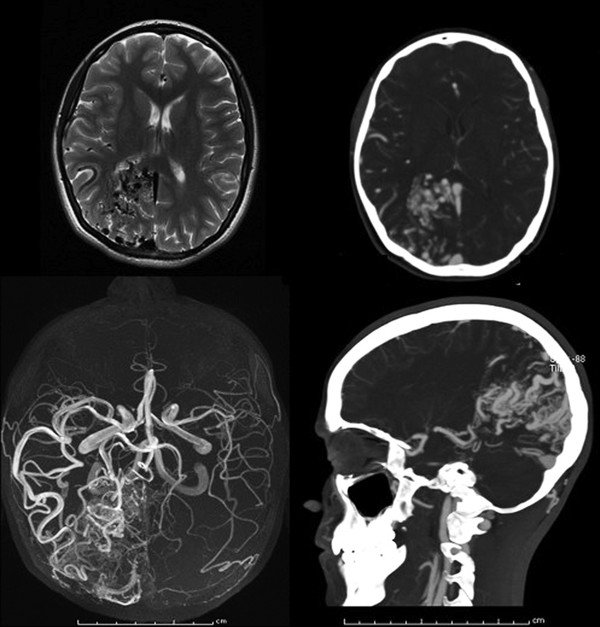
Fig. 67.1 Characteristics of an arteriovenous malformation on magnetic resonance (MR) imaging and computed tomography (CT). Upper left: Axial T2 MR image with dark flow voids evident in the right occipital pole. Upper right: Same lesion detailed by CT angiography. Lower right: Sagittal view of the lesion, demonstrating a wedge-shaped configuration. Lower left: A collapsed MR angiogram.
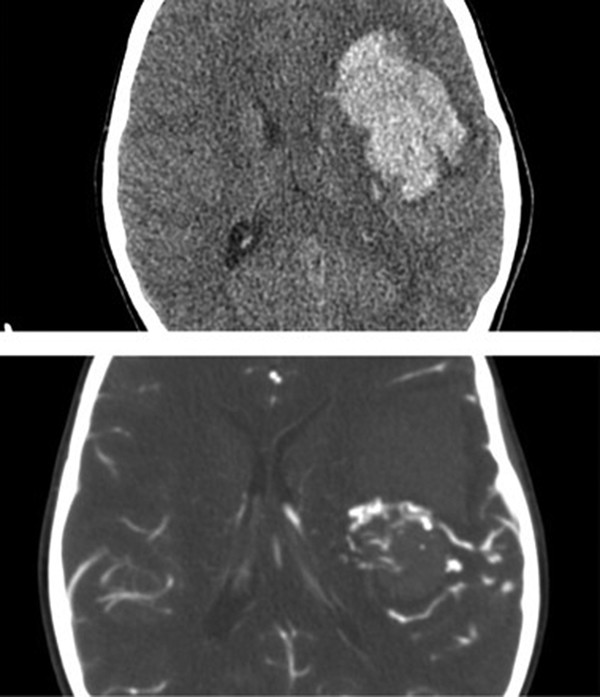
Fig. 67.2 Utility of computed tomographic (CT) angiography in an emergent setting. Upper image is a noncontrast axial CT scan showing a large left frontal hemorrhage. Lower image is a CT angiogram of the same patient revealing the relationship of an arteriovenous malformation to clot and serving to increase the safety of emergent clot removal.
Infratentorial AVM typically appears as a heterogeneous area of mixed density with serpiginous areas of enhancement after the infusion of contrast material. Cerebral atrophy may sometimes be seen on the affected side. A large malformation or an intracerebral hematoma may distort the normal intracranial anatomy. A hematoma from AVM can be situated in the cortex or adjacent white matter, so that it usually can be distinguished from a hypertensive hematoma, which is often deeply placed. Intraventricular extension of the hematoma is common.
MR imaging is also useful in the diagnosis and delineation of the anatomy of AVM (▶ Fig. 67.1). MR imaging better localizes the parenchymal structures relative to the AVM, even if there is a strong suspicion of AVM based on the CT or CT angiographic findings and angiography is planned. The typical MR imaging appearance is that of a latticework of signal-void spaces highly contrasted against the surrounding cerebral tissue on both T1- and T2-weighted sequences. These are intermixed with regions of various signal intensities corresponding to blood products in different stages of evolution and occasionally calcium and hemosiderin.37,38 The serpiginous shape of the vessels may be distinctive, identified as flow voids, and the relevant anatomy can be well visualized with MR angiography. Susceptibility imaging will sometimes disclose evidence of previous hemorrhage as a dark “bloom” around the nidus.39 Chronic ischemic changes, presumably a result of the “steal” phenomenon or venous hypertension, may be identified on MR imaging as bright signal of the surrounding brain on FLAIR (fluid-attenuated inversion recovery) or T2 images. The understanding of local ischemia can also be improved with diffusion–perfusion imaging.40
Traditional digital subtraction angiography remains the definitive investigative technique for the evaluation of intracerebral AVM (▶ Fig. 67.3). It establishes the nature and extent of the lesion, in addition to its blood supply and venous drainage.41 Angiography generally includes bilateral injection of both the internal and external carotid arteries and the vertebral arteries in order to visualize all of the vessels supplying the AVM. Three-dimensional angiography with computer-generated reconstruction is increasingly employed to depict lesional anatomy. It is important to underscore that 15% of cerebral AVMs receive some blood supply from the ipsilateral or contralateral meningeal arteries.42
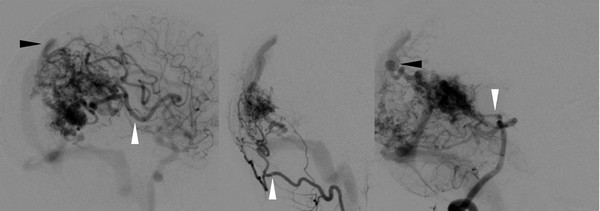
Fig. 67.3 Angiographic characteristics of arteriovenous malformation (AVM). Catheter angiography of the AVM from ▶ Fig. 67.1 reveals feeding arteries (white arrowheads) and draining veins (black arrowheads). Images include (from left to right) internal carotid artery, external carotid artery, and vertebrobasilar injections. These studies highlight the need to perform multiple-vessel injections to identify all potential lesional vessels.
The typical angiographic appearance of an AVM is that of distended, tortuous afferent and efferent vessels connecting with a tangled vascular mass, through which the circulation time is rapid (i.e., arteriovenous shunting). Angiography can fail to demonstrate an AVM despite suggestive findings on CT or MR imaging, usually because of the partial or complete thrombotic occlusion of feeding vessels. Other suggested causes of an angiographically occult AVM include small size, compression by adjacent clot, and destruction by hemorrhage. In the setting of a intracerebral hematoma without a clearly proximate cause, repeated MR imaging at 6 weeks after a hemorrhage is often recommended to screen for an occult AVM.35,36
For those patients presenting with seizure, electroencephalography can be considered during the initial evaluation. Electroencephalography may show focal or lateralized abnormalities that suggest the presence of an underlying structural lesion. These findings should prompt further investigation, most commonly MR imaging.
67.6 Indications for Surgical Treatment
67.6.1 Rationale for Intervention
The reasons for treating an AVM, particularly in children, are compelling. With each hemorrhage from an AVM, there is an approximately 25% chance that a permanent deficit or death will result, and children have an annual risk for hemorrhage of about 2%, a substantial cumulative risk for younger individuals with an expected long life span.43 Treatment has the potential to reduce or eradicate the lifetime risk for hemorrhage and also has the potential to eradicate AVM-related comorbid conditions, such as seizures and neurologic deficits. The goal of therapy is complete obliteration of the AVM. Partial destruction does not confer protection from bleeding in the future.33
In general, there is a consensus that intervention for patients with symptomatic lesions—particularly AVMs that have bled—is warranted. More difficult are those lesions that are found incidentally or that cause questionable symptoms. In these cases, the indications for treatment rest on weighing the risk of intervention against the risk of the natural history of the unruptured AVM. As discussed above, the risk for hemorrhage is incompletely understood, although some data suggest that AVMs in younger children are more likely to bleed over the course of a lifetime, increasing the impetus to treat. In addition to these studies, one can review the specific angioarchitecture of an individual lesion. Recent work has revealed that angiograms of pediatric AVMs can identify features that are associated with an increased risk for bleeding. Smaller AVM size, exclusively deep venous drainage, and an infratentorial location are specific angioarchitectural factors independently associated with an initial hemorrhagic presentation in children with AVMs, and the presence of these factors, even in an asymptomatic patient, may help to inform the decision to treat in selected cases.44
The optimal management of AVMs is controversial in both adults and children. AVM can be treated by one of three modalities—resection, endovascular techniques, or radiosurgery—or with a combination of embolization and either radiation or surgery. Each has inherent benefits and limitations. For some complex lesions, such as those with a high (4 or 5) Spetzler-Martin grade (see below), careful observation may be the appropriate option. The wide variety of lesional anatomical features and patient risk profiles, coupled with the lack of standardized outcome measurements, makes comparisons among studies difficult, reflected by the minimal information provided in the recent American Heart Association guidelines for the management of stroke in children.45 Outcome measures include the following: (1) radiographic obliteration of the lesion; (2) observer outcome scales (Rankin Scale or Glascow Outcome Scale); (3) patient-generated quality-of-life data; and (4) rates of specific comorbid conditions (e.g., seizures, headaches). The objective of this section is to offer guidance on when to select surgical treatment and how to provide the best operative management of these lesions.
67.6.2 Goals of Surgical Treatment
The operative goal is complete removal of the lesion. The decision to resect an AVM is based on several factors: (1) eloquence of the cortical location (speech, motor function, sensation); (2) pattern of venous drainage; (3) size; (4) associated aneurysms; (5) recent hemorrhage; (6) clinical deterioration; and (7) risk for complications from other modalities of therapy (e.g., radiation injury to the developing brain).46,47 Several of these factors are combined in the Spetzler-Martin48 grade, which incorporates eloquence of location, pattern of venous drainage, and size and is considered predictive of the outcome of surgical management (▶ Table 67.1).
| Size | Points |
| 0–3 cm | 1 |
| > 3–6 cm | 2 |
| > 6 cm | 3 |
| Location | |
| Non-eloquent cortex | 0 |
| Eloquent cortex | 1 |
| Deep venous drainage | |
| Not present | 0 |
| Present | 1 |
67.6.3 Indications for Surgical Treatment of Simple Lesions (Low Spetzler-Martin Grade)
A series of 20 pediatric patients with Spetzler-Martin grades 1 to 3 AVMs were treated by resection.49 Good recovery was achieved in 18 of 20 children (90%), and 1 death (5%) was related to prior hemorrhage. In addition to the clinical findings, radiographic outcomes were also evaluated; the radiographic obliteration rate was 89% (17 of 19). In another study, lesions with a low Spetzler-Martin grade were successfully treated by resection, whereas high-grade lesions were treated by radiosurgery.47 Other investigators have also recommended resection for low-grade or small, surgically accessible lesions, citing the benefits of immediate protection from rebleeding and the avoidance of delayed radiation-related brain injury. At the same time, they caution about the need for further study of the indications for surgical intervention.50
The safety and efficacy of resection are increased by preoperative embolization in selected patients, especially both children and adults with larger lesions.51–54 Preoperative embolization can reduce blood loss, shorten operative time, occlude vessels less accessible to the surgeon (e.g., deep feeding arteries), and decrease the size of the AVM.33 Nevertheless, no prospectively controlled studies have compared resection with and without embolization.
Although randomized trial data are lacking, the combined literature of institutional experience strongly supports resection as a primary treatment for children with Spetzler-Martin grade 1 or 2 AVMs. The low postoperative morbidity rates in patients with low-grade lesions (ranging from 0 to 12%), along with high rates of complete obliteration (up to 100%), suggest that delayed control, inherent to radiosurgery, may not be warranted.24,47,49,55 For comparison, a similar group of patients treated with radiosurgery alone had a reported 80% efficacy rate of lesion obliteration at 36 months, with 4 of 53 patients having recurrent hemorrhage after treatment.56
67.6.4 Indications for Surgical Treatment of Complex Lesions (High Spetzler-Martin grade)
Complex lesions (as defined by a higher Spetzler-Martin grade) carry a substantially greater risk for children. Multimodality therapy of AVM has been advocated by several investigators.24,47,57,58 Neurointerventionalists, radiation oncologists, and neurosurgeons work together to determine the best strategy for a particular patient. With the use of a multimodality approach, angiographic obliteration rates of 92.9% have been reported. In carefully selected patients treated with resection alone, a cure rate of 100% has been achieved.47
The decision process becomes controversial for patients with high-grade lesions. Preoperative embolization has been employed to decrease flow and occlude deep feeding vessels, maneuvers particularly helpful to facilitate resection. Embolization is rarely employed as the sole treatment for AVM, although the agent Onyx (Micro Therapeutics [Covidien], Mansfield, MA) has recently been used.50,59 Radiosurgery alone seems to protect against bleeding following radiographic obliteration. Others have argued that there is some protective effect even before total radiographic obliteration of the lesion.60,61
In one report, outcomes following resection with or without adjunctive embolization were 100% excellent or good at 36 months.47 These results are possible because some patients were excluded if they were considered to be poor surgical risks. Embolization can reduce the size of an AVM, making it more amenable to radiosurgery.59 In patients treated with radiosurgery without embolization, overall outcomes of 91% excellent or good and mortality of 9% were attained. When embolization was used before radiosurgery, the outcomes improved to 100% excellent or good at 36 months.47 The efficacy of the multimodality treatment of large, complex lesions is supported by the results in a group of 53 children, in whom a 58% cure rate was noted at 3-year follow-up for those with AVMs larger than 6 cm in diameter.62
In summary, the high likelihood of obliteration, coupled with low complication rates, makes a convincing argument in favor of multimodality treatment of pediatric AVMs. It is our practice at Boston Children’s Hospital to review cases in a multidisciplinary meeting to weigh the risks and benefits of intervention for AVMs. In general, high-grade lesions are treated only after a detailed review and a discussion of alternatives and risks with the family. It is not unusual to recommend observation for children with larger, high-risk AVMs (often defined as those with a Spetzler-Martin grade of 5 or 6).
67.7 Surgical Treatment
Once the decision to operate has been made, a series of steps are followed that involve the timing of the operation, perioperative management, and specific operative strategies. Although the marked variations in the size, location, and presentation of AVMs in the pediatric population preclude the possibility of a single, stock approach to all lesions, core principles remain that can reduce the risks of surgery. These key points are summarized in this section.
67.7.1 Timing of Treatment
The urgency of treating an AVM depends on its presentation. In the case of asymptomatic lesions, treatment planning can proceed in an elective fashion because the hemorrhagic rate is relatively low, approximately 1 to 3% per year for an unruptured AVM. In the case of lesions that have bled, rebleeding rates are approximately 6% for the first 6 months and 3% per year afterward.20,63
The treatment of an AVM that has already bled depends on the clinical findings and on the anatomy of the malformation. Elevated intracranial pressure may preclude definitive imaging of the lesion. Urgent surgical intervention is necessary if the child is acutely ill from a focal clot or from hemorrhage-related hydrocephalus. If formal angiography is not possible (because of the clinical presentation), then more rapid imaging of the vasculature, such as with CT angiography, is necessary for preoperative planning.64
The surgical alternatives are decompression of the cranial vault by evacuation of clot and/or cerebrospinal fluid diversion to lower intracranial pressure. In such emergent situations in which the anatomy of the lesion in unknown, given the low rebleeding rates of the lesions, definitive treatment of the AVM can be postponed until the child is clinically stable and the complex anatomy of the AVM can be delineated by formal angiography. Care should be taken to remove clot only, with the vascular malformation left undisturbed. Surgical treatment is usually scheduled at a later time (days to weeks) after ictus, often after better imaging has been obtained.
Preoperative embolization of an AVM assists the surgeon by reducing the size and flow of the lesion. In addition, deep arterial feeders in difficult locations can be treated before craniotomy. Embolization should be scheduled within a short period of time, 0 to 72 hours, before the resection. Longer delay risks the formation of new feeding vessels and collateralization, which can jeopardize resection. (For the same reasons, a similar schedule should be applied for embolization before radiosurgery.)
67.7.2 Perioperative and Anesthetic Considerations
The risk for an AVM bleeding during the induction of anesthesia is unknown, but it is probably low.33,65 Proximal arterial AVM-related aneurysms may increase the risk for bleeding due to shifts in blood pressure. Therefore, the goal should be normotension and euvolemia throughout the period of anesthesia.
Perioperative antibiotic administration for 24 hours is recommended. The use of antiepileptic medications and corticosteroids is debatable. Confirmation of complete resection by angiography is recommended, either intraoperatively or in the immediate (24 hours) postoperative period. New postoperative neurologic deficits should be investigated promptly by CT (to assess for hemorrhage or hydrocephalus) or by diffusion-weighted MR imaging (if ischemia is suspected).66
There are concerns specific to the pediatric patient undergoing resection of an AVM. Infants have a physiologically small blood volume; they cannot tolerate even minimal blood loss during a procedure. In addition, the cardiac output can be severely altered.67–69 Intravenous (IV) lines should be secured before the operation, and packed red cells must be in the operating room.
At Boston Children’s Hospital, we employ a standardized protocol for the operative management of children with AVM. Although it may not suit every institution, or every case, we offer it here as an example of a framework that minimizes variation and error (see box “▶ Operative Management of Children with Arteriovenous Malformation”).
Operative Management of Children with Arteriovenous Malformation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








