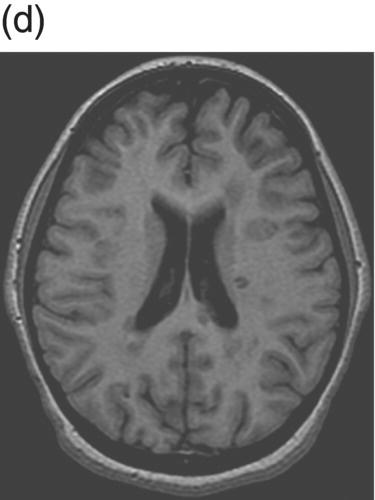
8 Robert Thompson Stone1 and Brenda Banwell2 1 Department of Neurology, University of Rochester Medical Center, Rochester, NY, USA 2 Department of Pediatrics (Neurology), The Hospital for Sick Children, Toronto, Ontario, Canada Once felt to be extremely rare, awareness of pediatric-onset multiple sclerosis (MS) has grown substantially over the last two decades. Over this time, a myriad of studies have emerged evaluating natural history data, diagnostic considerations, treatment safety, and effect on cognition. The condition differs from adult-onset disease in that the brain is actively myelinating and the immune system is actively maturing concurrent with the disease process. Children with an initial demyelinating event are more likely than adults to ultimately have a monophasic course. Thus, differentiation between events that will be relapsing versus monophasic can be challenging. There is also a more variable spectrum of clinical presentation in children, especially under age 12. This chapter emphasizes the clinical, neuroimaging, epidemiologic, and treatment aspects of pediatric-onset MS and summarizes current means to distinguish MS from other forms of acquired demyelinating disease. In 2013, the International Pediatric Multiple Sclerosis Study Group (IPMSSG) revised consensus definitions for acquired demyelinating syndromes (i.e., acute disseminated encephalomyelitis (ADEM), clinically isolated syndrome (CIS), neuromyelitis optica (NMO), and pediatric MS). ADEM is defined by polyfocal neurological deficits accompanied by encephalopathy (profound irritability, impaired level of consciousness, or coma). Patients may experience concurrent optic nerve and spinal cord involvement. Acute central nervous system (CNS) infection, metabolic disease, and other etiologies for acute CNS decompensation must be excluded. The diagnosis of ADEM also rests on the clinical and radiologic improvement (albeit not necessarily complete) following the inciting event. As the time course of improvement is variable, the current definition of ADEM permits a period of 3 months during which clinical and radiologic findings may evolve. Radiologic features typical of ADEM are described in section “Diagnostic Considerations”. Over 85% of children with ADEM will experience a monophasic illness with complete or near-complete neurological recovery. Children with spinal cord lesions and children requiring intense care are more likely to experience residual deficits. Multiphasic ADEM is defined as a second episode of ADEM, occurring more than 3 months from the initial illness, but not followed by any further events. If more than two demyelinating events occur, it would be considered a chronic disorder (most typically MS or neuromyelitis optica). Distinguishing multiphasic ADEM from MS rests on the presence of polyfocal neurological deficits and encephalopathy during both attacks and on the absence of clinically silent new lesions detected on MRI scans performed between events. Approximately 5–15% of children with an initial attack meeting criteria for ADEM will ultimately be diagnosed with MS. The diagnosis of MS in a child with an initial ADEM event could be made if a new demyelinating event occurs in the absence of encephalopathy, 3 or more months after the initial event, and is associated with new radiologic findings that meet the 2010 Revised McDonald criteria for dissemination in space. CIS is defined as an initial acquired demyelinating syndrome with mono- or multifocal neurological deficits in the absence of encephalopathy. It should not meet the criteria for a diagnosis of MS based on baseline radiologic features. Pediatric MS is defined by onset (first attack) prior to 18 years of age. Over 95% of pediatric MS patients experience a relapsing–remitting disease onset with multiple episodes of CNS demyelination separated in time (more than 30 days between attacks) and involving multiple areas of the CNS. An initial single nonencephalopathic episode with radiologic findings consistent with the 2010 Revised McDonald criteria for dissemination in space and time could also lead to the diagnosis. The majority of adult patients with an acquired demyelinating event will ultimately be diagnosed with relapsing–remitting MS. The proportion of children diagnosed with MS after an initial attack may be lower relative to adults, likely owing to the higher frequency of ADEM in childhood. In one pediatric cohort, after 3 years of follow-up, 57% were diagnosed with pediatric MS, monophasic ADEM was diagnosed in 29%, and 14% experienced monophasic CIS. A more recent study followed a cohort of children with acute demyelinating syndrome (ADS) for 3 years and utilized the IPMSSG definitions for classification. After the follow-up period, the patients were diagnosed with pediatric MS in 21%, monophasic disease in 76%, and relapsing demyelination other than MS (i.e., recurrent optic neuritis (ON), transverse myelitis (TM), or NMO) in 3%. Only 5% of children initially diagnosed with ADEM were subsequently diagnosed with MS. The risk of developing MS after an episode of childhood ON appears to be about 10–35%, with MS more likely to be diagnosed in children who have demyelinating lesions outside the optic pathways. Longitudinally extensive TM as an initial episode of ADS is less likely than the other forms of CIS to represent the first attack of MS. Overall, the incidence of ADS appears to be between 0.5 and 1.5 cases per 100,000 children per year, while the incidence of pediatric MS is approximately 0.3–0.5 per 100,000 children per year. Prevalence data for pediatric MS is limited. It is commonly cited that 3–5% of all patients with MS present with their first attack during childhood, the majority of whom manifest between ages 10 and 18 years. Onset of MS prior to age 10 years is rare, occurring in only 20% of all pediatric MS patients. The mean age of onset for pediatric MS is 12–13 years, whereas for monophasic ADS the mean age of onset is younger, that is, at 8–9 years. The female/male ratio in adolescent-onset MS patients is approximately 2–3:1, while male and female patients are equally represented when MS onset occurs prior to age 11 years. Hormonal influences on immune cell behavior or sex-specific expression of MS-relevant genes are postulated but as yet unproven explanations. In North America, pediatric-onset disease appears overrepresented in Black- or Asian-descent individuals. It has been observed that a high proportion of pediatric-onset MS patients are first-generation North Americans (a world region of high MS prevalence), while their parents often emigrated from world areas where MS is rare. These observations support a strong contribution of childhood environment on MS risk. The pediatric MS population is a useful cohort in which to study environmental risk factors given that children experience MS onset in close proximity to the pathobiological factors relevant to disease etiology. Remote infection with Epstein–Barr virus (EBV) is associated with MS in both pediatric and adult cohorts. Remote infection with EBV (as evidenced by seropositivity for anti-EBNA-1 antibodies) can be detected in 75–90% of children with MS. However, EBV infection cannot be obligatory given that 10–25% of patients have no evidence of exposure to EBV. Given that MS prevalence is highest in world regions with low ambient sunlight exposure, decreased exposure to sunlight and consequent low serum vitamin D concentrations are potential environmental risk factors for the development of MS. Children with MS have lower vitamin D concentrations compared to healthy children, and lower serum vitamin D concentrations detected at the time of a first demyelinating attack are predictive of subsequent MS diagnosis. There are many similarities between the clinical manifestations of pediatric and adult MS; however, the two populations are inherently different in that the pediatric brain is undergoing myelination and development concurrent with the MS disease process. Pediatric MS is relapsing–remitting in over 95% of cases, and thus, the discussion will focus on this form of disease. In very young children, an ADEM-like presentation occurs more commonly than in older children and adolescents. Ataxia, motor dysfunction, and brainstem deficits are the most common presenting signs and symptoms. Sensory disturbances as initial manifestations are far less common, which may be a function of the difficulty a younger child has in describing them. In older children, the presentation is most commonly monosymptomatic without encephalopathy, and sensorimotor symptoms as well as ON are the most common initial presentations. Cognitive impairment, fatigue, mood alterations, spasticity, bladder dysfunction, and tremor are features that may persist distinct from relapses. There are several rare tumefactive MS variants that can occur in the pediatric population including Marburg variant, Balo concentric sclerosis, and Schilder myelinoclastic diffuse sclerosis. Only the latter entity is more common in children. It typically presents with subacute onset of headache, encephalopathy, and motor or cerebellar dysfunction in the latter half of the first decade. MRI shows extensive bilateral lesions that may have a tumefactive appearance. The symptoms and brain lesions are characteristically exquisitely sensitive to corticosteroids, but disease activity can recur and progress over time. The interval between the initial demyelinating event and subsequent relapse is variable, with a range from 1 month (the minimum time interval delineated by diagnostic criteria) to over 30 years. There is some evidence that younger onset disease is associated with a longer interval to second attack. However, there is conflicting evidence to suggest that the interval between attacks in pediatric-onset disease is shorter than in adults. Overall, the mean annualized relapse rate in the first 2 years is 2–3, and about half to two-thirds of patients diagnosed with pediatric MS will relapse within 1 year. Once the diagnosis is established, longitudinal studies have cited annualized relapse rates of 0.3–0.9. Earlier onset in childhood is more likely than adolescent onset to be associated with polysymptomatic presentations accompanied by encephalopathy; however, most young patients have full recovery or only mild sequelae. There is intense interest in establishing predictors of disease course severity. This information is not only important for clinical decision-making but also critical for families struggling with the psychosocial consequences of such a diagnosis. Factors associated with relapses or with a risk of early disability include female sex, shorter interval between the first and second attack (less than 1 year), absence of encephalopathy at onset, characteristic MRI (see MRI discussion in the later text), less than full recovery after the first attack, and progressive disease at onset. Regarding time to secondary progression, a good general estimate is provided by using 10-year epochs. Patients with pediatric MS tend to reach secondary progression 10 years longer into their illness than those with adult MS but at an age 10 years younger. Pediatric-onset MS patients tend to enter into secondary disease progression (defined by progressive disability in the absence of clear relapses) 15–30 years after onset of disease, and this occurs at 30–40 years of age. In a large cohort study, the median time to reach Disability Status Scale (DSS) scores of 4, 6, and 7 occurred after 20, 28.9, and 37 years and at biological ages of 34.6, 42.2, and 50.5 years, respectively. This study also found that although the interval to secondary progression was longer in pediatric MS, once this phase was reached, the rate of disability accumulation was similar to that in adult patients. There are no universally accepted MRI criteria for pediatric MS, and many studies are based on adult criteria. However, it is clear that pediatric patients, especially those under age 10, can have different MRI characteristics. In fact, the 2001 McDonald criteria for lesion dissemination using baseline scans alone were found to be only 50% sensitive in a cohort of pediatric MS patients and were even less sensitive in the youngest patients. Children have a higher percentage of enhancing lesions on initial MRI (70% vs. 20% in adult MS) and a greater proportion of infratentorial lesions (70% vs. 30% in adult MS). There is also an increased incidence of larger, tumefactive lesions in younger patients. Table 8.1 summarizes the MRI criteria that have been proposed to aid in differentiating ADEM from pediatric MS. Pediatric MS patients are more likely to have T1-weighted hypointense lesions (black holes) and T2 hyperintense lesions with periventricular predominance. Initial MRI in pediatric MS tends to have smaller lesion loads and more distinct lesion borders (Figure 8.1 and Figure 8.2). Lesions secondary to ADEM are more likely to either completely or partially resolve over time, whereas children with MS accrue new, clinically silent lesions. Table 8.1 MRI Features on Baseline Scan That Predict Subsequent MS Diagnosis in Children Figure 8.1 MRI in ADEM. (a) Axial FLAIR image showing bilateral cerebellar and brainstem lesions with patchy, indistinct borders. (b) Axial FLAIR image showing left thalamic and occipital lesions. (c) Axial FLAIR image showing large hyperintense lesions that are patchy and without periventricular predominance. Figure 8.2 MRI in pediatric MS. (a) Axial FLAIR image with distinct, small brainstem hyperintense lesions. (b) Axial FLAIR image with numerous hyperintense lesions that have a periventricular predominance and distinct borders. (c) Axial T1 contrast-enhanced image showing a small left juxtacortical contrast-enhancing lesion. (d) Axial T1 imaging with several periventricular T1 hypointense lesions (black holes) that correlate with image (b). The cerebrospinal fluid (CSF) characteristics of pediatric-onset disease are much like those in adults. There is frequently a mild lymphocytic pleocytosis with normal or slightly elevated protein. Children may be less likely than adults to have oligoclonal bands (OCBs), although the available data is limited by variable techniques for OCB measurement. One study using rigorous methods demonstrated that 92% of pediatric MS patients have CSF OCBs, a finding very similar to the prevalence of OCBs in adult-onset MS. Children with ADEM or NMO are typically negative for OCBs, although this is not absolute. Similar to adult-onset disease, visual evoked potentials are more helpful than somatosensory and brainstem auditory potentials in aiding diagnosis. Around 50% of patients will have abnormal visual evoked potentials at the time of first attack, and nearly two-thirds of those patients will have no clinical evidence of ON. Other inflammatory, infectious, toxic, and neoplastic conditions should be considered when evaluating a child with acute neurological deficits. Inflammatory conditions (including systemic lupus erythematosus, Behçet syndrome, Sjögren syndrome, and sarcoidosis), although rare in the pediatric age group, can mimic features of MS. Headache, encephalopathy, cranial neuropathy, spinal inflammation, ON, and seizures can occur. Systemic involvement such as uveitis, oral and/or genital ulceration, dry eyes and mouth, arthralgia or arthritis, and rash should prompt appropriate investigations. Infections such as tuberculosis, human immunodeficiency virus (HIV), human T-lymphotropic virus (HTLV), and neuroborreliosis can mimic demyelination. CNS lymphoma can mimic the radiological appearance of MS but is rare in childhood. Vascular entities such as migraine, moyamoya syndrome, and primary CNS angiitis should be considered. While neurological deficits referable to vascular territories distinguish vasculopathy from MS, children with isolated small-vessel vasculitis typically have both clinical features and MRI findings that can be very difficult to distinguish from demyelination. Brain biopsy is required for diagnosis. Metabolic disorders, including leukodystrophies; mitochondrial diseases; amino and organic acid disorders; and peroxisomal disorders should be evaluated in children with symmetric MRI lesions, multisystem abnormalities, developmental disability, and progressive symptoms. Primary progressive MS is extremely rare in children. Table 8.2 highlights several conditions more common in pediatric patients.
Pediatric Multiple Sclerosis
Introduction
Definitions
ADEM, and multiphasic ADEM
ADEM
Multiphasic ADEM
Clinically isolated syndromes (CIS)
Pediatric multiple sclerosis
Clinical outcome following a first demyelinating attack
Epidemiology and risk factors
Clinical presentation
Disease course
Diagnostic considerations
MRI
KIDMUS criteria
White matter lesions located perpendicular to the long axis of the corpus callosum
Sole presence of well-defined lesions
100% specificity
11% sensitivity
Callen diagnostic criteria
At least two out of three of the following:
52% specificity
82% sensitivity
Callen MS versus ADEM criteria
At least two out of three of the following:
95% specificity
75% sensitivity
Verhey et al. criteria
93% specificity
84% sensitivity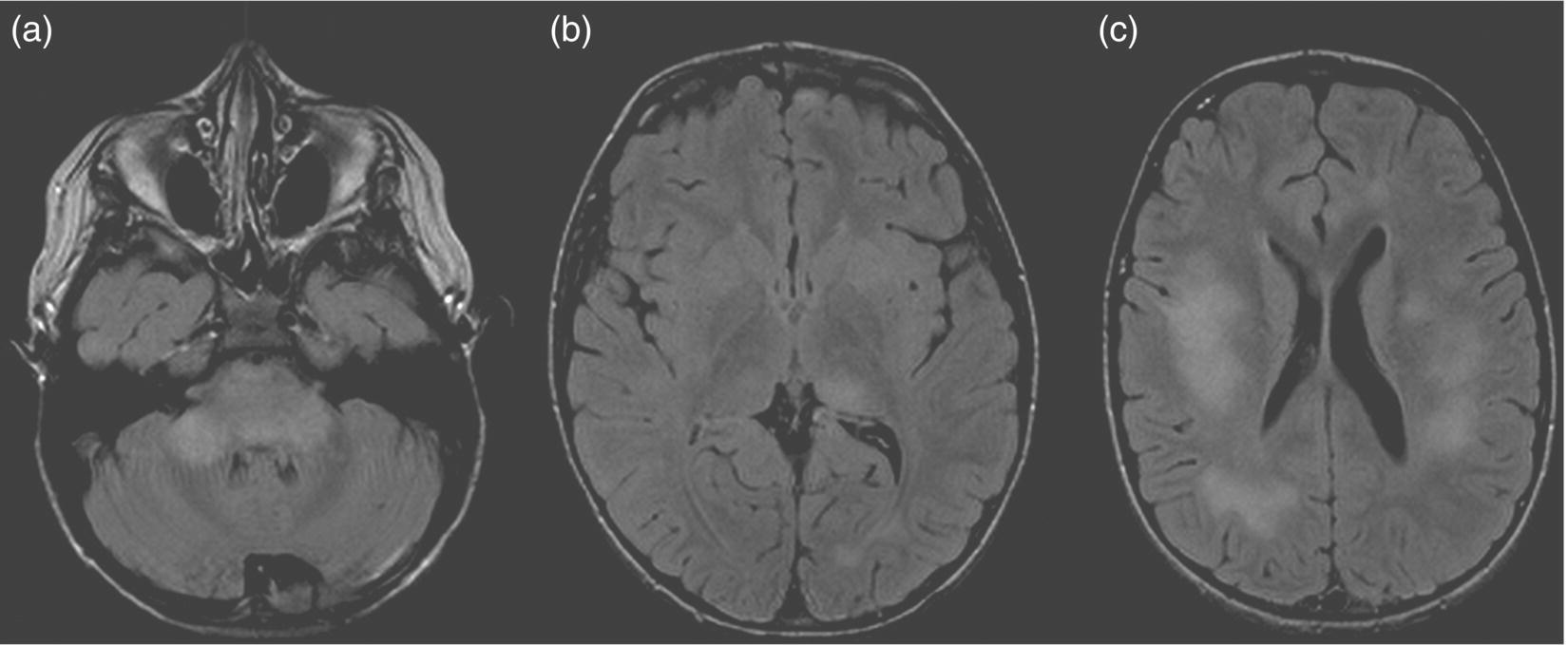
Spinal fluid
Evoked potentials
Differential diagnosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



