Pediatric Neurosurgery in Developing Countries
Many physicians who have worked in developing countries, even for a brief time, have been impressed by the great numbers of children who present with advanced stages of hydrocephalus (HC) and other diseases (▶ Fig. 5.1). The most commonly encountered condition is infant HC, which if untreated leads to death by the age of 2 years in 50% of cases and to significant disability in the majority of survivors; nonetheless, most children with HC in sub-Saharan Africa (SSA) go untreated. The reasons are multiple, but one reason is the lack of neurosurgeons in the region. There are around 100 neurosurgeons spread across SSA.1 If all infants with hydrocephalus had access to care in the centers where these neurosurgeons practice, the average annual case load for a neurosurgeon would be at least 1,000 operations per year if infant HC were all he or she treated, not counting additional operations for shunt failure and infection—clearly an untenable situation. In addition, families typically have very limited means for travel, and in regions with poor infrastructure or security concerns, travel can be dangerous as well as difficult.
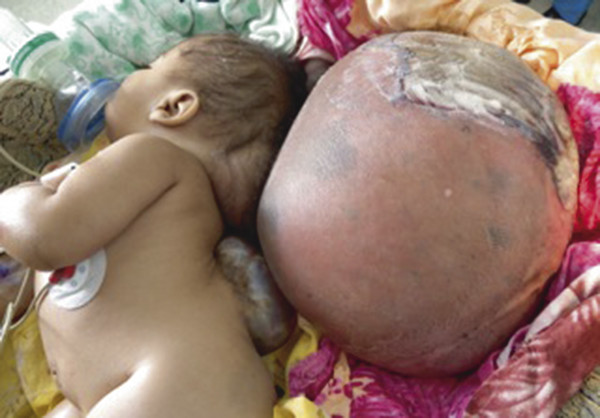
Fig. 5.1 A 7-month-old child who presented with untreated occipital encephalocele and thoracic myelomeningocele.
Before being brought to a medical center, many children are taken to a “practitioner of traditional medicine,” who may treat them with herbs or with focal cautery in the region of the abnormality (▶ Fig. 5.2). Families able to access a center with neurosurgical capabilities may be frustrated by their inability to pay for the cost of treatment, which typically includes purchasing a shunt. Because the cost of even a baseline shunt is prohibitive for many families, some surgeons insert either a length of Silastic tubing, tying off the distal end and making cuts just about the tie to create “slit valves,” or insert a valveless infant feeding tube. Such shunts are economical and considered by some to be effective, particularly in treating postinfectious HC.
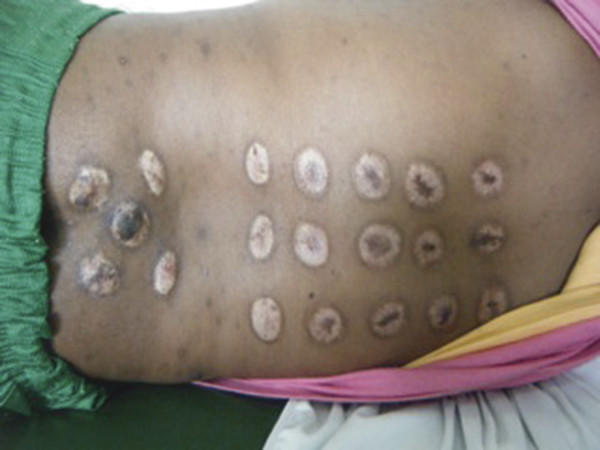
Fig. 5.2 Thoracolumbar scars resulting from the treatment of back pain secondary to a spinal–intraspinal tuberculoma by a practitioner of traditional medicine.
A further obstacle to treatment in these children is a lack of general awareness in regard to the nature of hydrocephalus and the fact that it is a treatable condition. In areas where HC treatment has not been previously available, the public should be made aware of the condition of infant HC, what it looks like, and where to get help. Depending upon the local culture, it may be appropriate to emphasize that this is a treatable medical condition, not the result of spiritual forces. Experience in Uganda demonstrated that this may be done effectively through newspaper and radio informational announcements, the result of which was a gradual decline in the age and disease severity of patients presenting for treatment over several years.
This speaks not only to the burden of disease, but also to the lack of access to diagnosis and treatment. In SSA, the annual number of new cases of infant HC can be minimally estimated to be more than 90,000, and potentially more than 300,000.2 We recently reported that, after the use of a mortality-adjusted minimal estimate of annual cases of infant HC in SSA (82,000), the annual economic burden is at least 1 billion U.S. dollars, and possibly as much as to 56 billion U.S. dollars (depending upon the economic methods employed).2 In the same study, we demonstrated that the most conservative estimate of the benefit-to-cost ratio for treating HC is 7:1, with an upper bound estimate of 50:1.
5.1 Congenital Lesions
5.1.1 Hydrocephalus
The most common cause of infant HC throughout SSA is unknown, and the causes may vary regionally or have differences in referral patterns. A previous report suggested congenital causes of HC to be most common.4 At CURE Children’s Hospital of Uganda (CCHU), we found ventriculitis to be the most common cause of infant HC, accounting for 60% of the cases, with three-quarters of the infections occurring within the first month of life as a component of neonatal sepsis.5 We have presented evidence suggesting that Acinetobacter and related species may be the dominant infectious agents.6 This is in contradistinction to neonatal sepsis and meningitis in developed countries, where group B streptococci and Escherichia coli predominate. We have also demonstrated that in Uganda, infection peaks are tightly linked to rainfall patterns.7 Similarly, at Kijabe Hospital in Kenya, the most common etiology of HC was reported to be prior infection among all patients older than 3 months; however, for those younger than 3 months at treatment, myelomeningocele was the more common etiology.8 Thus, it is possible that many cases of infant HC in this region might ultimately be avoided. Public health strategies for the effective prevention and treatment of neonatal sepsis are sorely needed.
Clinical Presentation
Whether postinfectious or associated with spina bifida, HC is most common in infants, who may present with head circumferences ranging from normal to more than 60 cm, and it is accompanied by the same symptoms and signs that are seen in developed countries. Infants are often anemic and malnourished, and they have higher risks for infection and poor wound healing after shunting. Computed tomography (CT) and magnetic resonance (MR) imaging are available in few centers; ultrasonography is more widely available. Ultrasound images permit measurements of the lateral and third ventricles; the fourth ventricle is seen less commonly unless it is grossly abnormal.
The ethical issue of whether to treat severe infantile HC is a recurring one. Infants who are not treated develop progressive macrocephaly, sometimes to head circumferences of 80 cm, and many die of aspiration pneumonia or malnutrition within 2 years. However, some infants live for several years, and their caregiver burden is enormous. Insertion of a ventriculoperitoneal (VP) shunt or treatment with endoscopic third ventriculostomy and choroid plexus cauterization (ETV/CPC) may have no benefit on the neurologic condition of some infants with severe HC, but the prevention of severe macrocephaly has considerable benefit for the caregivers—not only because of the reduced weight of the infant head but also because of fewer pressure ulcers in the parietal regions.
Medical Treatment
At times, the treatment of severe HC is futile. If insertion of a VP shunt is thought to carry unacceptable morbidity because of malnutrition, poor home environment, or ventriculitis, and if cerebrospinal fluid (CSF) production cannot be diminished by CPC, medical treatment with acetazolamide may be considered. Doses of 50 mg/kg per day have been reported to significantly diminish CSF production for up to 1 month; the longer-term effects are unknown.8
Treatment with Shunts
In the best of circumstances, shunts are expected to fail at least once in the first few years after placement. In the North American Shunt Design Trial, 61% of patients were free of shunt failure at 1 year and 47% at 2 years, with a shunt infection rate of 8.1%.9 Even though some have reported higher shunt complication rates for SSA,10 others have reported outcomes comparable to those in North America. In a prospective study of 195 consecutive children at CCHU with 90.3% 1-year follow-up, 54.5% of patients survived to 1 year with no shunt complications (including infection, wound complication, and shunt malfunction), with all shunt malfunctions occurring within the first 3 months.11 In that study, the shunt infection rate in the first year was 9.7%. A subsequent survival analysis of 900 first-time shunt placements at CCHU demonstrated the infection rates at 30 days, 90 days, 6 months, 1 year, and 2 years to be 5.7%, 9.5%, 11.7%, 13.3%, and 15.2%, respectively.16 It was notable that although the majority of known infections occurred in the first 6 months, late shunt infections continued to occur over the 7-year time span of the study at a rate of about 1% per year. A retrospective analysis of 574 patients at Kijabe Hospital in Kenya, with a mean follow-up of 8.9 months in 76% of patients, demonstrated that 65% had no shunt complications within the first 2 years, and shunt infection was diagnosed in 9.1%.6 However, prospectively collected data in Kijabe indicate a current infection rate of 15 to 20%. In both of these institutions, the shunt predominantly used was the inexpensive Chhabra shunt, for which no difference was found, in regard to rates of shunt failure or infection in the first year, when it was compared in a randomized prospective trial with a shunt commonly used in North America that costs 20 times more.11 Thus, it is encouraging that even when a very inexpensive shunt system is used, the expected outcome for shunt treatment in SSA may not be substantially different from that in more developed countries. Higher shunt complication rates would be expected because of previously cited factors, such as malnutrition, thin skin, anemia, and concurrent illnesses.
Ventriculoscopic Treatment
Rationale for Its Use in a Developing Country
Despite the potential for reasonable shunt outcomes in SSA, the obstacles to treatment access discussed above eliminate the safety net for shunt maintenance that we rely on in more developed nations. Once the fontanels of a child with a shunt are closed, an emergency shunt malfunction in a rural, “up country” location may end in disaster. For this reason, at CCHU, we aggressively pursued ETV as the primary treatment.3 We noted a lower risk for infection for ETV (< 1%) compared with our shunt infection risk of around 10%. Also, most ETV failures occur within the first 6 months of treatment, when the fontanel is open and treatment failure tends to be visually obvious and less precipitous—in contradistinction to shunt failures, which continue to occur over the lifetime of the patient and can lead to rapid clinical deterioration.
Combined Endoscopic Third Ventriculostomy and Choroid Plexus Cauterization
The initial experience with ETV was encouraging, but we found, as have others, that young age increases the risk for ETV failure.3 Acting on the hypothesis that this was the result of decreased CSF absorption among infants with congenital causes of HC (e.g., aqueduct stenosis, fourth ventricular outlet obstruction), we began including lateral ventricle CPC bilaterally along with ETV to treat the residual “communicating” HC12(▶ Fig. 5.3). We found that for infants younger than 1 year of age, the addition of CPC significantly increased the overall success of ETV from 47% to 66% (p < 0.0001). The operative technique employed for the combined ETV/CPC procedure has been previously described in detail.12,13
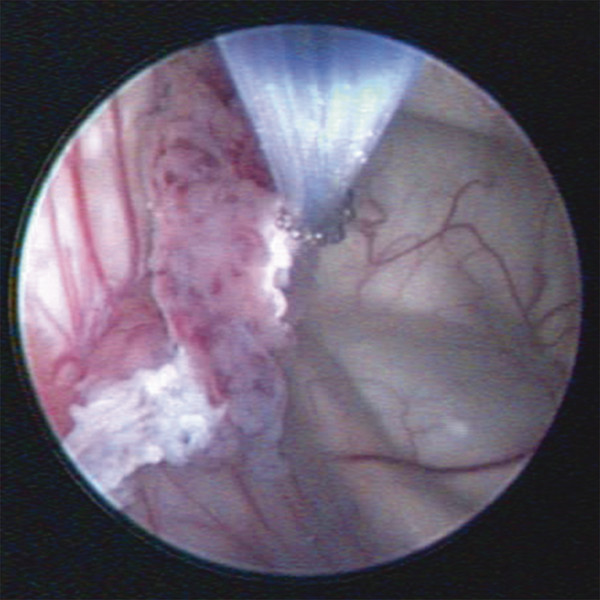
Fig. 5.3 Cauterization of choroid plexus in the lateral ventricle.Monopolar electrocauterization was performed with a Bugbee wire.
We subsequently demonstrated the efficacy of the combined ETV/CPC procedure for subsets of infants with HC of distinct etiologies, including postinfectious hydrocephalus (63.3%), myelomeningocele (76% success), encephalocele (79% success), Dandy-Walker complex (74%), and congenital aqueduct stenosis (81.9%).12–17 It is notable that the median age at the time of ETV/CPC in these reports was 2.0 months for infants with myelomeningocele, 3.0 months for infants with postinfectious HC, and 3.1 months for those with congenital aqueduct stenosis. Thus, these results are relevant for infants younger than 3 months of age. For infants with postinfectious HC, the outcome for ETV was more affected by the status of the aqueduct (open or closed) and the prepontine cistern (scarred or open) than by whether CPC was performed.12,14 Cisternal scarring is an especially good predictor of failure in these patients, more than doubling the risk for failure after control for other factors.18 Based upon our first attempt at ETV in 1400 patients, we were able to construct a success prediction score based upon patient age, the etiology of HC, and the extent of CPC.19 We are working to refine this by using the additional parameters of aqueduct and cistern status.
Mode of Hydrocephalus Treatment and Developmental Outcome
We were concerned that despite the potential benefits of being shunt-independent, there might be some developmental advantage to the treatment of HC by shunt placement, given the general impression that the ventricles tend to remain larger after ETV than with shunt placement. However, in a spina bifida cohort, we were unable to detect any difference in early childhood development between children treated endoscopically and those treated with shunts.20 Thus, there was no apparent developmental advantage for HC treatment with shunts that would dissuade us from continuing to pursue ETV/CPC as our primary treatment option.
Mode of Hydrocephalus Treatment and Survival
Our original impetus for pursuing endoscopic treatment was the assumption that shunt dependence would increase mortality in the long run, and thus its avoidance would actually save lives. However, we demonstrated no significant difference in 5-year survival between children treated by shunt placement and those successfully treated by ETV or ETV/CPC for myelomeningocele or postinfectious HC.21 The majority of deaths occurred from non–HC-related causes (mostly infectious diseases and malnutrition), and they were sufficiently common to obscure any small difference in survival that might have existed between the treatment groups. It was concerning that for these two groups of children, all of whom had some degree of disability, either from the myelomeningocele/Chiari type 2 malformation or the initial brain injury resulting from the neonatal infection causing postinfectious HC (which results in varying degrees of cerebral palsy), the 5-year survival was significantly less than that for their unaffected peers in Uganda. The 5-year survival of Ugandan infants in the general population is reported to be 84% (a child mortality rate of 16%). In the survival studies we conducted at CCHU, we found the 5-year survival for infants treated for postinfectious HC, myelomeningocele, and encephalocele to be 70.5%, 63%, and 61%, respectively.13,15,16 The increased mortality may have been related to several factors, including reduced access of disabled children to routine medical care, unrecognized symptoms of HC, or lack of care by their families. We found that the only factor favoring 5-year survival for the children with spina bifida was referral to a community-based rehabilitation program that included regular home visits. These children had significantly better 5-year survival (80%), which was equivalent to that of their unaffected peers.16 Patients who were not enrolled in one of these programs were three times more likely to die.
Effect of Prior Endoscopic Treatment on Subsequent Shunt Survival
We were concerned that for those patients in whom ETV was abandoned for shunt placement or for those undergoing shunt placement in the face of a successfully completed, but failed, ETV (± CPC), there might be an increased risk for shunt infection or obstruction as a result of the endoscopic procedure. However, in a retrospective study of 900 consecutive shunt placements at CCHU, we found no difference in risk for shunt infection or failure among patients who received a shunt primarily, at the time of an abandoned ETV (± CPC), or following a completed but failed ETV (± CPC).22
Economic Impact of Primary Endoscopic Treatment
In the CCHU experience, in which 60% of patients have postinfectious HC, the ETV attempt has to be aborted in about 30% of patients because of anatomical distortion (primarily from prior ventriculitis in those with postinfectious HC). Nonetheless, about half of all patients presenting for treatment are successfully treated with a single endoscopic operation and avoid shunt dependence. The average total financial cost of endoscopic treatment at CCHU in 2005 was $670, and the cost of initial shunt placement was $470 (when the inexpensive Chhabra shunts were used, each of which costs less than $40).22 However, with a conservative estimate of two anticipated shunt revisions over time, and ignoring the additional cost for shunt infection, the total cost of initial placement and subsequent maintenance of shunt function was determined to be $1,545 over time.22 Avoiding this cost in 50% of patients by attempting endoscopic treatment in all patients would create a potential savings of $43,750 for every 100 patients presenting for treatment, an appreciable amount in resource-poor settings.
Training
Given the apparent advantages of attempting endoscopic treatment for HC as the primary method, we have advocated the training and equipping of neurosurgeons in developing countries in these techniques. The level of sophistication and skill required by the variable and sometimes difficult anatomy and the medical fragility of many of these infants have compelled us to focus our training efforts on neurosurgeons, although we have successfully trained nonneurosurgeons from regions with no neurosurgeons in certain very select cases. Through CURE Hydrocephalus, a division of the Christian nongovernment organization CURE International, fellows in neurosurgery are trained at CCHU, and after successful completion of their training, their home institutions are provided with the necessary equipment to develop their own programs. CURE Hydrocephalus also hires a local HC clinical coordinator for patient follow-up, given our reported observation that a home visit program may independently reduce mortality.16
Conclusion
It is unrealistic to expect long-term successful treatment of infant HC in developing countries with the model of visiting surgical teams that place shunts but are unable to provide follow-up or future access for the maintenance of shunt function. An argument can be made for placing the highest priority on training and equipping centers to provide competent longitudinal care. Furthermore, a strong case can be made for a policy of endoscopic treatment as the primary intervention, either by intention to treat in all patients or by refining the selection criteria according to established predictors of ETV success. The primary treatment of infant HC by ETV/CPC is more likely to be effective than ETV alone, and this treatment paradigm can dramatically reduce the incidence of shunt dependence—an especially important outcome in the context of countries with limited resources. This approach does not appear detrimental to developmental outcome, nor does it increase the subsequent risk for shunt malfunction or infection for those children in whom a shunt is ultimately required. The majority of ETV/CPC failures occur within the first few months of surgery, during a relatively safe period of infancy when failure is visibly obvious to the family and is not an acute emergency. Furthermore, a paradigm of intention to treat by endoscopy may be more cost-effective in a given population. The increased mortality we have observed in disabled children can be mitigated with community-based rehabilitation or home visit programs, which should ideally be a component of any regional HC treatment center in the setting of a developing country.
5.1.2 Meningoceles and Myelomeningoceles
The incidence of meningocele (MC) and myelomeningocele (MMC) in developing countries appears to be significantly greater than that in developed countries, partly because of the lack of folic acid in diets, partly because of the ingestion of maize contaminated with the mycotoxin fumonisin (a folate antagonist),23 partly because of the lack of prenatal screening and abortion, and partly because of the high birth rates in developing countries, such that the number of any congenital abnormalities would be increased. Approximately 300 infants come to Kijabe Hospital each year with untreated MMC. Few mothers of infants with MMC have heard about the benefit of folic acid in reducing the risk for MMC; they are given a year’s supply of folic acid when their baby is discharged.
MC in developing countries seems to occur in the same ratio to MMC (~1:20) as in developed countries and to have the same presentation, imaging characteristics, treatment, and outcomes as in developed countries. However, MMC has a different spectrum; infants seem to have higher lesions, to have a greater likelihood of paraplegia or of an associated kyphus or split-cord malformation, and to be born with substantial HC.
Developing countries have few, and sometimes no, facilities to care for infants with MMC. Neurosurgeons and multidisciplinary care are rare, and if care is provided, it is often by a general surgeon who closes the back and/or inserts a VP shunt. Because children with MMC are a substantial drain on family resources, they are often not taken for treatment, and many die within a few months. Some centers insert a shunt but do not close the MMC, thinking that the baby’s death would be the best outcome for both it and the family, and that in those infants who do survive, epithelium and then scar tissue will develop over the MMC.
MMC is rarely detected prenatally. Many deliveries occur at home or in rural hospitals, and transfer to facilities where care is available may be delayed because the family has insufficient funds to pay for the transportation, much less the medical care. Thus, although most children with MMC present within 2 months after birth, some do not present until 2 to 3 years later. When infants present within a few days after birth, some MMCs have ruptured. Even those that are intact may have become infected. In Kijabe, we analyze CSF from both the MMC sac and the ventricles on admission. CT or MR imaging is rarely available (or needed) before closure of the MMC, but lateral spine X-rays are helpful in evaluating kyphotic deformities (▶ Fig. 5.4).
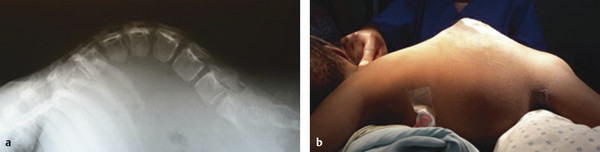
Fig. 5.4 Lateral spine radiographs of (a) an infant with a congenital kyphotic deformity associated with a myelomeningocele and (b) a 2-year-old child with a myelomeningocele–kyphotic deformity that was unrepaired at birth.









