Pediatric Spinal Vascular Malformations
Spinal vascular malformations are a distinctly rare but important pathologic entity in the pediatric population. From low-flow lesions like cavernous malformations to larger, high-flow lesions like juvenile arteriovenous malformations, significant variability occurs that requires a neurovascular team approach to management. Although these conditions are quite rare, with some estimates of less than 1 in a million persons,1 the morbidity associated with the disease process can be significant. Presentations in the child population are often acute and dramatic.2–4 However, with appropriate management, patients can be treated with potential cure and improvement of neurologic status.
The first description of spinal vascular malformations was made by Virchow in 1865.5 At that time, they were felt to be neoplasms of the spine. The first surgery for a spinal vascular malformation, in a 13-year-old boy, was reported in 1912 by Charles Elsberg.6 The patient’s condition did not improve after surgery. Later attempts concentrated on dural arteriovenous fistulas with venous stripping, but successes were relatively few. A spinal vascular malformation was first imaged in 1927 by Perthes, who used Pantopaque myelography.5,6 A true understanding of this class of disorders and their anatomy began with the use of spinal angiography in the 1960s.
In this chapter, we discuss the different anatomical types of spinal vascular malformations, highlighting their diagnosis and treatment. We also address syndromes that can be associated with these malformations. It should be noted that numerous classification systems have been used for spinal vascular malformations, mostly based on anatomical considerations. The most frequently used systems divide vascular malformations with shunting into four types: type I, spinal dural arteriovenous fistula (AVF); type II, intramedullary arteriovenous malformation (AVM); type III, juvenile diffuse AVM, or Cobb syndrome; and type IV, perimedullary AVF (▶ Fig. 70.1). Spetzler et al have recently added the conus AVM as a separate entity.7 Type I spinal dural AVF, although the most common type in adults, is an acquired lesion and is not found in children with any frequency.3 Cavernous malformations (CMs) are discussed in detail separately.
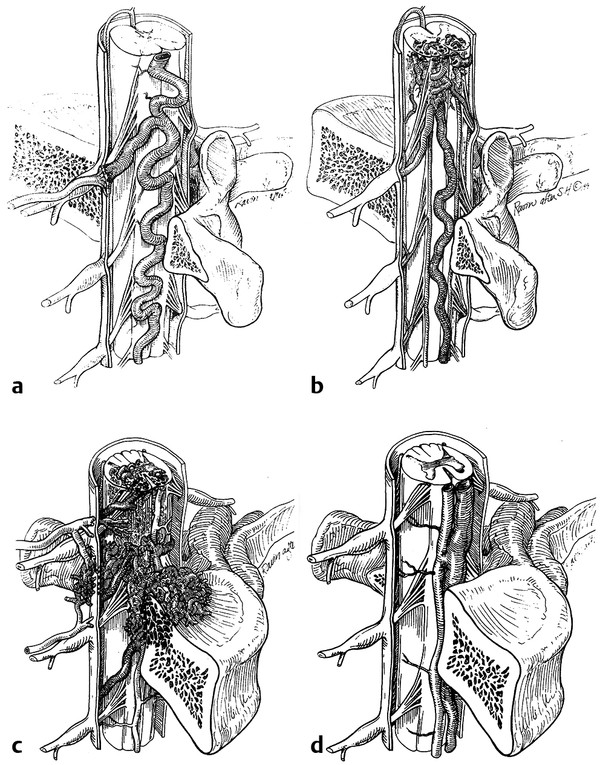
Fig. 70.1 Schematic representation of the classic four types of spinal vascular malformations.
(Reproduced with permission from the American Association of Neurological Surgeons, Rolling Meadows, IL.)
70.1 Spinal Intramedullary Arteriovenous Malformations
Intramedullary AVMs (type II) are true intramedullary malformations with a nidus interposed between multiple feeding arteries and draining veins that involve the substance of the spinal cord.6 Often referred to as glomus AVMs, these lesions are high-flow and high-pressure systems with an arterial supply derived from the anterior and/or posterior spinal arteries (▶ Fig. 70.2).
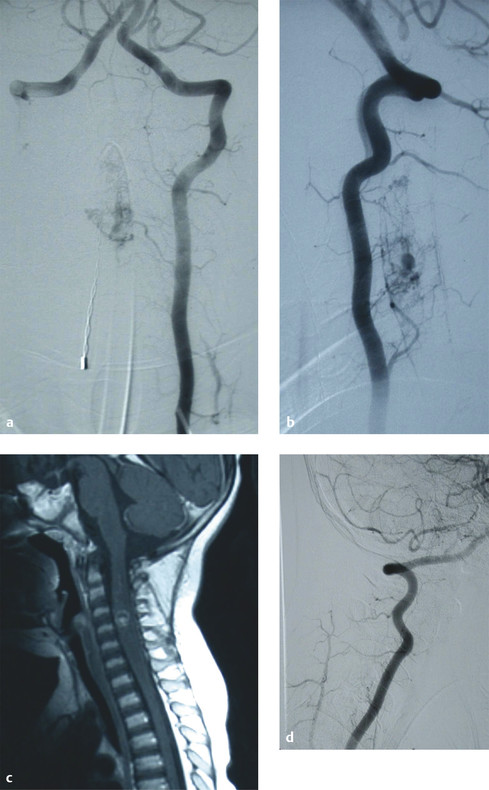
Fig. 70.2 (a,b) Preoperative angiograms demonstrating a cervical intramedullary arteriovenous malformation (AVM). (c) Preoperative magnetic resonance image illustrating that the AVM is intramedullary. (d) Postoperative angiogram showing complete obliteration of the fistula.
Uncommon in the general population, spinal AVMs in children account for a small percentage of the cases of this rare disease.3,8 The morphology of the malformations does not differ from that of the adult lesions per se, but the presentation and morbidity can differ. These are important clinical entities because they produce considerable morbidity and may even be fatal if left untreated.
Treatment strategies are unique because pediatric diseases carry a complex set of variables that must be incorporated into treatment planning. In the later pediatric presentation, the disease features tend to more closely resemble those of the adult disease process, so it is postulated that the early pediatric presentation may be a severe form of the adult disease process.2
Like perimedullary fistulas, juvenile-type AVMs in the pediatric population are seen more often in boys than in girls.2,3 Pediatric intramedullary AVMs more often present with a history of progressive and fluctuating myelopathy and with episodes of acute neurologic deterioration secondary to hematomyelia.2,3 The lesions often involve the dorsal thoracic spine or conus medullaris.8 Some authors advocate placing conus medullaris AVMs in a distinct category.9
Unlike type III AVMs (spinal arteriovenous metameric syndrome [SAMS], or Cobb syndrome), which are extensive congenital vascular malformations, and type IV AVMs (perimedullary AVFs), which can be associated with syndromes like hereditary hemorrhagic telangiectasia (HHT), type II spinal AVMs are usually spontaneous.10
70.1.1 Incidence and Prevalence
Spinal AVMs are underdiagnosed in the general population because of their variable and complex presentation, resulting in an estimation of the incidence of the disease rather than the true incidence. Estimations of 3.3 to 11% of all spinal cord lesions and 10% of all central nervous system (CNS) AVMs are reported in the current literature on the subject.11,12 These estimations are based on single-institution experiences with relatively small sample sizes, in large part as a consequence of the rarity of the disease.
Disease incidence is less frequent in the pediatric population. Berand in 1972 retrospectively reviewed 304 cases of AVMs of the spinal cord and found that 1.9% of patients were younger than 10 years and that 13% were younger than 20 years.13 Rodesch et al14 reviewed 155 patients with CNS arteriovenous shunts between the years of 1981 and 1999 (single institution) and found pediatric spinal AVMs to account for 5% of all CNS AVMs and for 20% of AVMs of the spine. Of the four types, types II and III are more commonly diagnosed in children and young adults.
70.1.2 Natural History and Presentation
Given the rarity of this disease, it is not commonly considered in the differential diagnosis, especially because of the lack of specific presenting clinical symptoms. Symptoms reported in the literature include neck, chest, and back pain; sensory and motor deficits; bowel and bladder dysfunction; headache; altered mental status; meningeal symptoms; and spinal bruit.3,15–18 Children more commonly present with an acute onset of symptoms than do adults.3,10 A review of 72 cases of pediatric spinal vascular malformation found a 52.8% incidence of acute presentation versus a 30.6% incidence of chronic, progressive symptom presentation for intramedullary spinal cord AVMs.3 An acute presentation often is a result of hemorrhage, either hematomyelia or subarachnoid hemorrhage.3,8
The presenting symptoms can differ between extremely young children (younger than 2 years) and children younger than 15 years. Case studies of children younger than 2 years showed that they presented with a significantly lower incidence of hemorrhage, either hematomyelia or subarachnoid hemorrhage, than did children younger than 15 years.19 Cullen et al reported an incidence of hemorrhage of 23% in patients younger than 2 years with spinal AVMs versus an incidence of up to 67% in pediatric patients younger than 15 years.2
Initial estimates of disease progression and a poor prognosis for children with these lesions were likely influenced by the dural AVF literature.2,6 Unlike those who present with spinal cord AVFs, patients who present with hemorrhagic intramedullary AVMs usually improve without treatment. Cullen et al showed that 72% of pediatric patients improved, with only 20% showing worsening symptoms.2 Interestingly, recurrent hemorrhage was seen in only 9% of patients.2 These data support the concept that observation may be a reasonable option for patients in whom surgery is associated with unacceptable risk.
70.1.3 Treatment
The gold standard for the treatment of intramedullary AVMs remains microsurgical resection.6,8 Surgery can usually be approached with a posterior laminectomy or posterolaterally with a transpedicular exposure. General surgical outcomes for pediatric patients are better than those for their adult counterparts. A review by Kalani et al in 2012 reported the results of treatment of spinal AVMs at a high-volume center.8 Three of the patients were treated with surgery alone with good results.
Endovascular treatment provides an adjunct and, in some rare cases, an alternative to surgery for intramedullary and conus medullaris AVMs. If embolization is planned, some authors advocate spinal provocative testing to aid superselective angiography.8,20,21 In these cases, amobarbital (Amytal; Marathon Pharmaceuticals, Deerfield, IL) and lidocaine can be injected to test for clinical or neuromonitoring changes. If the test is negative, embolization can be completed with N-butyl-cyanoacrylate (NBCA) or Onyx (Micro Therapeutics [Covidien], Mansfield, MA). No clinical studies have compared the efficacy of embolization versus that of open microsurgical treatment.
At this time, the treatment for this complex lesion should begin with a high-quality spinal angiogram to assess its angioarchitecture.8,22 If there are pedicles to the AVM that can be traversed safely with a microcatheter without endangering the anterior or posterior spinal arteries, an embolization can be attempted.21 Complete cures by embolization have been reported, although they are in the minority.8,22 After embolization, the majority of patients require surgical resection. The recommendation for resection should be made on individualized case-by-case basis. Although complete resection is the goal, the surgeon must recognize the limits of the resection and identify vessel loops that may leave a permanent deficit if chased into the spinal cord parenchyma. Velat et al described the resection of these lesions with a subtotal pial resection method, which devascularizes the AVM without violating the spinal cord parenchyma.23 All patients should undergo postoperative angiography and, if possible, intraoperative angiography to document the extent of the resection.
Stereotactic radiosurgery currently does not have a treatment role in this patient population, considering the small size of the cord and the anticipated long life expectancy of the patients.10
70.2 Spinal Arteriovenous Metameric Syndrome/Cobb Syndrome
Spinal arteriovenous metameric syndrome (SAMS) is an extremely rare disorder that is nonheritable. It is characterized by a vascular malformation that extends beyond the spinal cord and involves the bones, paraspinal, skin, and soft tissues that arose from the same metamere24,25 (▶ Fig. 70.3). Cobb syndrome represents involvement of the entire metamere. The syndrome has previously been classified as a type III or juvenile AVM and is often referred to as an intradural–extradural AVM. Rodesch et al postulated that other genetic nonheritable syndromes, such as Klippel-Trénaunay syndrome and Parkes Weber syndrome, represent a lesser spectrum of these metameric disorders with multiple AV shunts.14
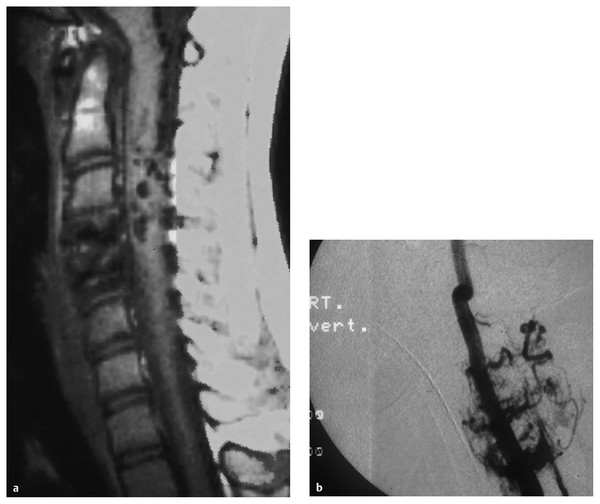
Fig. 70.3 (a) Magnetic resonance image and (b) angiogram showing a metameric spinal arteriovenous malformation.
70.2.1 Incidence and Prevalence
The actual incidence of Cobb syndrome is difficult to estimate. The first report of the syndrome was in 1890.25 It was not truly recognized until 1915, when Cushing deduced that Cobb had had a patient with an underlying spinal AVM in addition to the cutaneous findings. Overall, the complete metameric syndrome has been reported in fewer than 50 patients in the literature.24 Like other pediatric spinal malformations, it shows a male predilection.3
70.2.2 Natural History and Presentation
Cobb syndrome presents equally with acute and chronic symptoms.4,24,26 Classically, acute presentations have a rapid monoparesis or paraparesis with loss of bladder or bowel function.25,26 Chronic symptoms include progressive back or radicular pain, progressive weakness and numbness, and bladder and bowel involvement. The natural history of Cobb syndrome remains unpredictable, with disease rapidly progressing in some patients and stabilizing in others.10 There are currently insufficient data to identify the prognosis accurately, although of the 5 identified patients treated conservatively, 2 worsened, 2 improved, and 1 stabilized.24 In fact, Malis reported that “their rarity is perhaps the only favorable aspect of these lesions.”27
70.2.3 Treatment
The treatment of Cobb syndrome rarely results in complete resection of the AVM.8,24 Reports in the literature have described combined embolization and surgical resection resulting in complete cure, although they are rare.28,29 The goal for the treating physician should be to disconnect large feeding vessels with embolization and to combine this embolization with surgery to decompress the nerve roots and spinal cord, if possible.8 Often, embolization alone can reduce symptoms by decreasing venous hypertension and vascular steal. Additionally, some authors add corticosteroid administration and radiation treatment to surgery or embolization.30 Clark et al published a review of patients with Cobb syndrome.24 Seventeen patients underwent surgery alone, with seven reported improvements. Six underwent combined embolization and surgery, with five showing improvement. Overall, each case should be approached individually to assess what is best for the pediatric patient. Often, surgical resection should be avoided in a very young patient because of the potential for excessive blood loss.
70.3 Perimedullary Arteriovenous Fistula
Type IV AVMs are intradural, perimedullary, direct AVFs on the (usually ventral) pial surface of the cord between the anterior and/or posterior spinal arteries and the medullary veins (▶ Fig. 70.4). These fistulas were first described by Djindjian et al.31 Anson and Spetzler further subclassified them as types IV-A, IV-B, and IV-C based on their size and flow characteristics.7 Type A is a small fistula with minimal shunting and minimal venous hypertension, type C is a large fistula with high flow and significant venous ectasia, and type B is intermediate between type A and type C. Other authors have divided perimedullary fistulas into microfistulas (IV-A) and macrofistulas (IV-B and IV-C). A macrofistula in a pediatric patient is often correlated with HHT and may be the presenting manifestation of the disease.
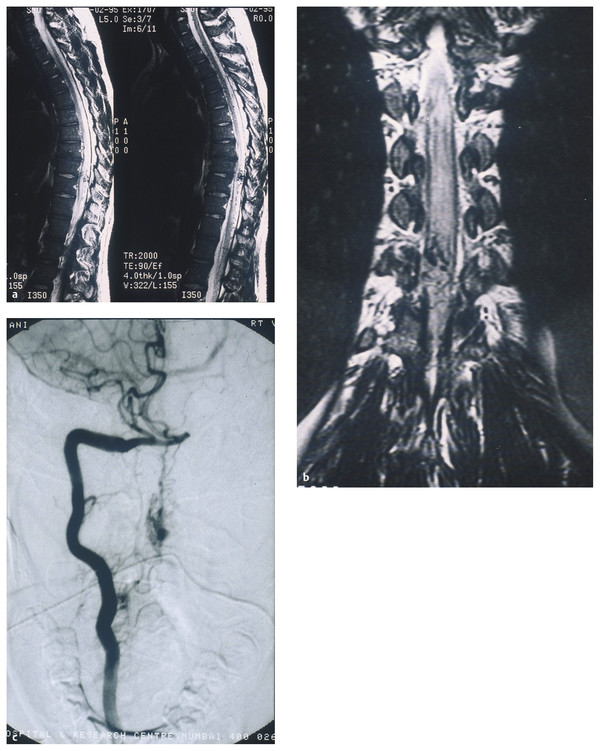
Fig. 70.4 (a,b) Magnetic resonance images showing a perimedullary fistula in a pediatric patient. (c) Angiogram confirming the diagnosis of perimedullary arteriovenous fistula.









