Pharmacogenetics and Pharmacogenomics
Nicole Walley
Sanjay M. Sisodiya
David B. Goldstein
Introduction
Although pharmacologic treatment successfully controls seizures for a majority of patients with epilepsy, current treatment options are far from satisfactory for many patients. Recent progress emerging from the genome project and related activities suggests that it may finally be possible to systematically search for genetic differences among patients that influence how they respond to treatment. Here we review the potential of pharmacogenetics to improve the treatment of epilepsy and address some of the barriers to progress.
Limitations of Current Epilepsy Treatment
Perhaps the most important weakness of current treatment options is that they fail to control seizures for a substantial minority of patients. Nearly one-third of patients do not achieve adequate seizure control upon treatment with any of the currently available antiepileptic drugs (AEDs).63 Furthermore, those who fail even one drug trial have a poor prognosis: Only 11% of patients who withdraw from their first AED due to inefficacy will ever go on to achieve seizure freedom.17,40,41 The end result is a significant number of patients who are forced to (a) live with recurrent seizures or (b) resort to invasive surgery, which is not available to all refractory patients, is not guaranteed to eradicate seizures, and can have devastating consequences in some cases.
Although nine new AEDs have been approved during the last 15 years, these have made only a modest impact on the proportion of patients who respond well to treatment. There are currently more than 15 pharmacologic agents now available for clinical use, but approximately 30% of epilepsy patients remain refractory to treatment.5,63 The chances of achieving seizure freedom with new AEDs has not been significantly improved, although levetiracetam (Keppra) is reported to reduce seizures in 40% of refractory patients, with 6% to 13% achieving seizure freedom.8,39,68
The other key limitation is that AEDs can cause both serious and life-threatening adverse reactions, as well as less serious reactions that may nonetheless have important effects on patient quality of life. It has been estimated that 17% of emergency room visits due to adverse drug reactions (ADRs),58 and 10% of ADRs leading to hospitalization87 are caused by AEDs. ADRs were cited as the cause of discontinuation in nearly 40% of decisions to terminate treatment with a particular medication40 and, although patients exposed to high doses of AEDs are more likely to develop ADRs, side effects are not necessarily dose related and also can occur during the course of efficacious drug trials.
The newer AEDs, although offering little improvement for refractory cases, do tend to be tolerated better by patients. Withdrawal rates due to adverse reactions are significantly lower for the newer AEDs.41 However, older ADRs have not been eradicated and some new ADRs have been introduced, such as the behavioral and cognitive ADRs associated with levetiracetam and topiramate respectively. Tolerability remains a major limitation in epilepsy treatment.
In addition to these key aspects, there are secondary challenges in the use of AEDs, notably the trial-and-error process usually required to identify appropriate doses or drug combinations for individuals. Patients require dramatically different doses to control seizures (Fig. 1), however efficacious dose is impossible to predict. Current routine, nonemergency methods of dosing stipulate, “start low, go slow.” A patient is slowly titrated upward until he becomes seizure-free or he experiences dose-related ADRs; at this point, use of a new drug is often considered. This can take months for those who require uncharacteristically high doses of a medication, or years for those who fail one or more trials and must repeat the process—during which time patients continue to suffer seizures.
Pharmacogenetics as a Probe into Biological Processes
In our view, the improvement of seizure control and the minimization of side effects are the primary motivations for pharmacogenetics studies in epilepsy, and projects should be prioritized on this basis. It is also worth noting, however, that pharmacogenetics may open a window into biologic process that are poorly understood in humans and otherwise not amenable to study.
Levetiracetam and Human Behavior
The approval of levetiracetam has brought seizure relief to some previously refractory patients. It is generally well tolerated, but has been known to cause behavioral abnormalities. Behavioral ADRs have been cited as the most common reason for withdrawal from levetiracetam treatment, and between 5.9% and 30.7% of patients report experiencing behavioral changes.35,86 Levetiracetam-induced behavioral abnormalities can manifest as irritability, aggression, depression, or psychosis. The identification of gene variants relevant to these ADRs therefore could identify pathways relevant to human behavior, and perhaps more specifically relevant to neuropsychiatric conditions such as schizophreniform psychosis.
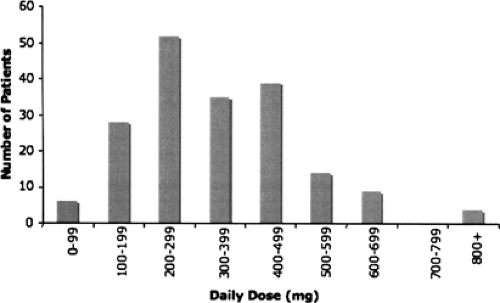 FIGURE 1. Distribution of maintenance dose of lamotrigine. Maintenance dose among patients from the same clinic is highly variable. All patients were treated with Lamo-trigine at the National Hospital for Neurology and Neurosurgery, Queen Square, London. Additional (maximum) dose distributions can be found in Tate et al.76 |
Topiramate and Human Cognition
Topiramate is a new AED commonly used as adjunctive and monotherapy. The most common reason for discontinuation of topiramate therapy is cognitive side effects, which can manifest as mental slowing, language difficulties, and confusion.
Nearly one-half of patients exposed to topiramate report cognitive ADRs (unpublished data), and 27% of patients who discontinue topiramate treatment cite cognitive adverse events as the cause of cessation.77 Although topiramate is most commonly used as add-on therapy, and the incidence of ADRs is increased with polytherapy, healthy volunteers challenged with topiramate alone also experience cognitive difficulties, as determined by neuropsychological tests.47,49 Although many AEDs have negative cognitive side effects, topiramate’s profile is distinctive. For example, verbal fluency is more affected by topiramate than by other AEDs24 and appears to affect only a subset of susceptible patients.51 If genetic differences that mediate to-piramate sensitivity exist among people, their identification may inform clinicians about the underlying brain systems involved in verbal fluency tasks.
Nearly one-half of patients exposed to topiramate report cognitive ADRs (unpublished data), and 27% of patients who discontinue topiramate treatment cite cognitive adverse events as the cause of cessation.77 Although topiramate is most commonly used as add-on therapy, and the incidence of ADRs is increased with polytherapy, healthy volunteers challenged with topiramate alone also experience cognitive difficulties, as determined by neuropsychological tests.47,49 Although many AEDs have negative cognitive side effects, topiramate’s profile is distinctive. For example, verbal fluency is more affected by topiramate than by other AEDs24 and appears to affect only a subset of susceptible patients.51 If genetic differences that mediate to-piramate sensitivity exist among people, their identification may inform clinicians about the underlying brain systems involved in verbal fluency tasks.
Epilepsy Pharmacogenetics: What is Needed?
Genetic Methods
It is possible (although still not certain) that systematic pharmacogenetic research can identify gene variants that will considerably improve the use of AEDs. Here we describe the key elements of contemporary pharmacogenetics research.
Candidate Gene Approach
Until recently, it was not feasible to consider in a single study more than a handful of genes; even then, the genetic information considered for each gene was very limited. Very recently, however, it became feasible to carry out large-scale candidate gene studies in which the genetic variation in hundreds of genes is systematically analyzed. Moreover, whereas whole-genome association studies remain costly, reasonable coverage of most polymorphisms in the full human genome is available in standardized sets of polymorphisms, such as those supplied by Illumina.
In pharmacogenetics, a particularly strong case can be made for candidate gene approaches because the likely modes drug action are usually known or suspected, at least partially. Therefore drug targets make obvious candidate genes and clearly deserve careful evaluation. Consistent with this notion, recent work on the targets of warfarin and phenytoin/carbamazepine identified polymorphisms associated with dosing.14,76 Moreover, nearly 80% of genetic variants identified by pharmacogenetics reside in the three major categories for pharmacogenetic candidates: Drug targets, drug metabolizing enzymes, and drug transporters (although this reflects in part the bias of where researchers have chosen to look; for a review of this subject see the work by Goldstein, Tate, and Sisodiya28 and their unpublished update.
For epilepsy pharmacogenetics, a set of high-priority candidate genes are readily identified on the basis of both pharmacokinetics and major modes of action.
Pharmacokinetic candidates
Drug-metabolizing enzymes (DMEs). The most studied genes to date in pharmacogenetics are those encoding DMEs. For most AEDs, the key enzymes that metabolize the parent compound and active (or toxic) metabolites are well known (Table 1), and many of them have well described functional variants. It is already clear that these variants have some impact on patient response to AEDs: For example, CYP2C9 variation affects phenytoin dosing (see discussion in next section). It does not appear likely, however, that DME variation will prove of substantial clinical relevance in epilepsy.
Transporters. Drug transporters may influence AEDs at a variety of points. They can influence uptake in the gut, the amount of metabolism in the liver and, perhaps most important, the properties of the blood–brain barrier. In the latter case, the effect of transporters may either be general or affected by seizures. As a class of proteins, however, drug transporters are not well studied. It is not clearly known what transporters move which drugs at therapeutically relevant concentrations, and few examples of functional variation have been characterized, making it unclear what role transporters play in pharmacogenetics.
Table 1 Drug-Metabolizing Enzymes of Commonly Used AEDs | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||
Table 2 Known and Suspected Mechanisms of Action of Commonly Used AEDs | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
Pharmacodynamic candidates
Drug targets. The major modes of action for most AEDs are known, although in some cases the most important among several candidate modes of action remains unclear (Table 2). The major modes of action fall into one of three broad categories: Modulation of voltage-gated ion channel function, enhancement of γ-aminobutyric acid (GABA)-mediated inhibition, and attenuation of excitatory (glutamate-mediated) transmission.42 In addition to
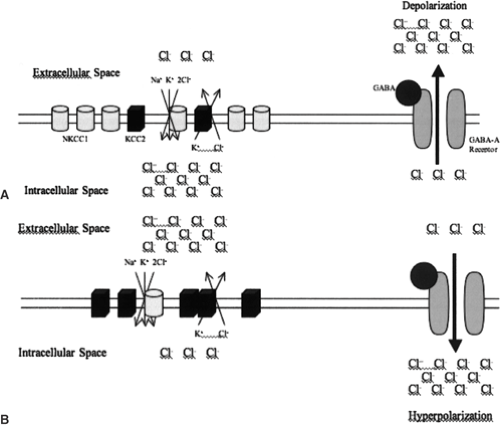
the precise target itself, it should also be appreciated that molecules downstream of the drug targets upon which an AED acts may contain variation that influences response. Thus, pharmacogenetic studies of GABA-acting drugs should appropriately consider chloride ion homeostasis broadly and consider relevant genes. The importance of this perspective is clearly illustrated by chloride ion transporters.
Chloride ion homeostasis is primarily regulated by a balance of oppositely acting chloride transporters: NKCC1 (also known as SLC12A2), which transports chloride ions into the neuron and KCC2 (also known as SCL12A5), which extrudes chloride ions from the neuron. In adult neurons, KCC2 is the dominantly expressed transporter, resulting in a high extracellular concentration of chloride ions. In fetal neurons, the balance is reversed, such that NKCC1 is dominantly expressed, and there is a high intracellular chloride ion concentration.54,61 The change in expression results in GABA having an inhibitory effect in mature neurons and an excitatory effect in fetal neurons (Fig. 2). This is known to have pharmacologic implications, because GABA-acting drugs can exacerbate seizure activity in infantile seizures.25 In addition, expression levels of these chloride channels are altered in seizure-affected brain tissue.52,67 From a pharmacogenetic standpoint, genetic alteration of chloride transporter protein expression or function most certainly has the potential to affect patient response to GABA-acting drugs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree