Pineal Region Tumors
39.1 Pineal Gland
The normal pineal gland measures only 5 to 10 mm. It is histologically composed of pinealocytes (95%) and glial cells (5%). Pinealocytes are a specialized form of neuronal cells and function as neuroendocrine transducers. In lower mammals, these cells exhibit structural characteristics of photoreceptor cells. In primitive animals such as fish and amphibians, the pineal gland is a neurosensory photoreceptor organ. In mammals, it has a neurotransmitter secretory function. The function of the pineal gland in humans is not well understood, however. The pineal gland is innervated by sympathetic nerve fibers that originate from the superior cervical ganglia. The pinealocytes play a role in translating the noradrenaline input released from sympathetic nerves into a hormonal input. The pineal secretory function, specifically melatonin and serotonin synthesis and release from the pinealocytes, has been studied extensively. Serotonin concentration in the pineal gland is highest in the brain. Melatonin release in animals follows a circadian rhythm, with high serum levels during the night and low levels during the daytime. Melatonin also has an inhibitory effect on the hypothalamic–pituitary axis and at the gonadal level.1 However, in humans, although melatonin levels are highest between the ages of 1 and 5 years and then decrease until the end of puberty,2 the function of melatonin remains unexplained.3
39.2 Pineal Region
The pineal region comprises multiple structures in and around the pineal gland of the posterior third ventricle. The pineal gland is bordered by the cerebrospinal fluid (CSF) space, the third ventricle anteriorly, and the quadrigeminal cistern posteriorly. The neural structures that surround the pineal gland are the quadrigeminal plate of the midbrain inferiorly, the cerebellar vermis posteriorly, and the splenium of the corpus callosum superiorly. Lateral to the pineal gland are the posterior thalami, and anteroinferiorly is the tegmentum of the midbrain, which is continuous anteriorly with the hypothalamus. The lateral wall of the third ventricle is traversed by a hypothalamic sulcus from the foramen of Monro to the aqueduct of Sylvius. This sulcus separates the thalamus above from the hypothalamus below. The roof of the third ventricle consists of the tela choroidea and fornix.
39.3 Pathology and Pathobiology
Heterogeneous types of tumors occur in the pineal region. Tumors of pineal and extrapineal origin differ in histologic type. Those of pineal origin are germ cell tumors and pineal parenchymal cell tumors. Extrapineal tumors arise from the surrounding neural or mesenchymal structures and include astrocytomas, meningiomas, ependymomas, and choroid plexus papillomas. Another tumor in this location with papillary features, papillary tumor of the pineal region, is considered to be derived from the subcommissural organ.4,5
39.3.1 Germ Cell Tumors
Tumors of germ cell origin are classified as germinomas, embryonal carcinomas, endodermal sinus tumors (yolk sac carcinomas), choriocarcinomas, and teratomas. Germinomas, which are the most common of the germ cell tumors, are composed of cells with suppressed differentiation potential. On the other hand, embryonal carcinomas are considered to be composed of pluripotential cells. They further give rise either to embryonal tumors, which consist of all three germ layers (i.e., mature teratomas and immature teratomas) or to extraembryonal tumors. The latter include choriocarcinomas through trophoblastic differentiation and yolk sac tumors through yolk sac formation. Of all the germ cell tumors, only the mature teratoma is considered to be benign. Other, nongerminomatous tumors are malignant and are included in a category of nongerminomatous germ cell tumors (NGGCTs). Elements of various germ cell tumors may coexist in a single tumor; this type of tumor is called a mixed germ cell tumor. Teratomas, on the other hand, are derived from all three germ layers of embryonal structures and are composed of well-differentiated tissues with an organoid pattern. Those composed of mature cells are benign teratomas. Immature teratomas, however, contain primitive elements derived from all or any of the three germ layers and behave in a malignant fashion.
The origin of germ cell tumors is controversial. Germ cell tumors derive from pluripotential germ cells and span a wide range of differentiation and malignant characteristics.6 The primordial germ cells appear in the yolk sac wall in the third gestational week. They migrate from the fetal yolk sac via the dorsal mesentery of the hindgut into the genital ridge in the sixth gestational week. They also migrate and disseminate widely throughout various tissues and organs in the early embryo. In the extragonadal location, these germ cells often remain in two midline sites: the mediastinum and around the third ventricle. This theory explains why extragonadal germ cell tumors frequently develop in the thymus and in the pineal and hypothalamic regions. However, in normal human anatomy, the primordial germ cells are not found in these locations. Sano theorized that misplaced embryonic tissues incorrectly enfolded at the time of neural tube formation become the source of intracranial germ cell tumors.7 Despite their early migration, these germ cells tend to develop neoplastic transformation at a much later stage of life, around puberty. One assumes that gonadotropins or gonadotropin-releasing hormones secreted by the hypothalamus at puberty may have a carcinogenic effect.8
Intracranial germ cell tumors show genomic alterations indistinguishable from those of their extracranial counterparts; these include gain of 12p and the X chromosome.9 Also, mutation of the c-KIT gene is common among germinomas.10
39.3.2 Pineal Parenchymal Tumors
Pineal parenchymal tumors are derived from pineal parenchymal cells within the pineal gland. These tumors possess the potential to differentiate into several cell lines, such as neuronal, astrocytic, ependymal, retinoblastomatous, and mesenchymal components. Pineal parenchymal tumors are classified into pineoblastoma, pineocytoma, and intermediate types, depending on the cellular differentiations.11 Pineoblastomas are poorly differentiated malignant tumors and belong to the group of primitive neuroectodermal tumors (PNETs). Pineocytomas, on the other hand, are differentiated and clinically benign. Some parenchymal tumors may have mixed components of pineoblastoma and pineocytoma. On immunohistochemical studies, Yamane et al reported variable immunopositivity among pineal parenchymal tumors when they used monoclonal antibodies against human pineal tissue, whereas glial differentiation seemed very rare.12 Ultrastructural studies found that neoplastic pineal cells appeared to differentiate either toward a neurosensory pathway characterized by the presence of sensory cell elements, such as vesicle-crowned rodlets and fibrous filaments, or toward a neuroendocrine pathway.13,14 Cytogenetic studies showed monosomy of chromosomes 20 and 22 and gain of 1q, 5p, 5q, 6p, and 14q.15 The gene patterns of the three pineal parenchymal tumors fell in the same cluster. The pineocytomas showed high expression of TPH, HIOMT, and genes related to phototransduction in the retina (OPN4, RGS16, and CRB3), whereas the pineoblastomas showed high expression of UBEC2, SOX4, TERT, TEP1, PRAME, CD24, POU4F2, and HOXD13.15 Papillary tumors of the pineal region showed high expression of SPEDF, KRT18, and genes encoding proteins reported to be found in the subcommissural organ, namely ZFH4, RFX3, TTR, and CGRP.15
39.3.3 Tumors of Glial and Miscellaneous Cell Origin
Astrocytes are normally present in the pineal gland, but pure pineal gland glial tumors are exceedingly rare. Nearly all glial tumors arise from the glial tissue elements intimately surrounding the pineal gland. Astrocytomas often originate in the thalamus or the midbrain and extend to the pineal region. Glioblastomas, astroblastomas, ependymomas, oligodendrogliomas, choroid plexus papillomas, and medulloepitheliomas may occur in this region. Among tumors of mesenchymal origin, meningiomas, hemangiomas, and cavernomas may occur in the pineal region.
39.3.4 Nonneoplastic Cysts
Pineal cysts, which result from focal degeneration of the pineal gland, contain gelatinous material. The cyst wall is composed of three layers: an outer fibrous layer, a middle layer of pineal parenchymal cells with variable calcification, and an inner layer of hypocellular glial tissue.16,17 Other developmental cysts include epidermoid and dermoid cysts. Arachnoid cysts consist of CSF and a cyst wall composed of arachnoid membrane. These cystic lesions may have a similar appearance on neuroimages.
39.4 Epidemiology
Pineal region tumors comprise 3 to 8% of all intracranial tumors among children.18 Pineal region tumors affect more Japanese and other persons of Asian origin.19,20
Intracranial germ cell tumors account for 0.4 to 3.4% of intracranial neoplasms in the United States and Europe.8 Germinomas are the most common variety among them, comprising 35 to 41%1,21 or 61 to 65%8,22,23 of germ cell tumors. Their average incidence in the United States is considered to be 0.1 per 100,000 persons per year.24 Germ cell tumors of the pineal region tend to affect males, with a male-to-female ratio of 4:1.8,21 Most germinomas occur in the first three decades, with a peak in the middle of the second decade, corresponding to the onset of puberty. About 65% occur between the ages of 10 and 21 years, and only 11% occur before the age of 9 years.
NGGCTs are highly malignant. Most cases of NGGCTs occur in males in the first two decades of life. Choriocarcinomas tend to occur at a younger age (mean age, 8 years) than do embryonal carcinomas and endodermal sinus tumors (mean age, 14 and 17 years, respectively). Of choriocarcinomas, 35% occur before the age of 9 years, whereas only 10 to 12% of embryonal carcinomas and endodermal sinus tumors occur before the age of 9 years.
Teratomas in the pineal region often affect males. Most teratomas occur in children younger than 9 years, but 20% occur in persons between the ages of 16 and 18 years.
The occurrence of mixed germ cell tumors has increased because of a recent trend of aggressive tumor biopsy and the availability of tumor marker studies. In a study by Matsutani et al, 49 (32%) of 153 intracranial germ cell tumors were of mixed type.21 The common components frequently present in mixed germ cell tumors are germinoma and teratoma, whereas a combination of germinoma and choriocarcinoma is very rare. The correct identification of mixed germ cell tumors and NGGCTs requires adequate tumor sampling and proper preparation of the tissue for immunohistochemical and electron microscopic examination.23
Pineoblastomas often occur in infancy and childhood,25 whereas pineocytomas occur in older children and young adults.11,14,26,27
The ratio of germ cell tumors to pineal parenchymal tumors was reported to be 3.6:1, whereas germ cell tumors accounted for 31% of the tumors in 282 patients of all age groups with pineal region tumors.28,29 In Japan, germ cell tumors are much more common than pineoblastomas, which comprise only 5.1% of pineal region tumors.30
39.5 Symptomatology
The common signs and symptoms of pineal region tumors are primarily related to increased intracranial pressure due to hydrocephalus, which is present in almost all cases. Hydrocephalus is the result of obstruction of the aqueduct of Sylvius. The most common symptom is headache. The headaches usually occur intermittently initially but become more frequent and intense. They are worse in the morning and often awaken the patient during sleep. Nausea and emesis are common signs in association with headaches. Double vision may be due to either abducens nerve palsy or tectal compression. Occasional blurred vision can be due to the tectal compression but can also be related to visual obscuration secondary to papilledema. In the later stages of hydrocephalus, patients exhibit an ataxic gait and altered mental status. Papilledema is a frequent sign of hydrocephalus secondary to pineal region tumors.
Other signs are related to direct compression of the neural tissue, particularly the quadrigeminal plate and pretectal region of the midbrain. Parinaud syndrome is well-known to be pathognomonic for pineal region tumors and is present in about 50 to 75% of patients with pineal region tumors. It is due to compression or structural damage on the posterior commissure. The sudden occurrence of the setting sun phenomenon and Parinaud syndrome together with decreased mental status may be related to hemorrhage into the pineal tumor (pineal apoplexy).
Synchronous germinomas in the pineal and suprasellar regions may present with diabetes insipidus and other hormonal dysfunctions and with visual impairment.31 Pineoblastomas and less often germinomas may have disseminated through the CSF pathway at the time of diagnosis, which may cause various signs such as spinal cord compression and optic nerve compression.32 Precocious puberty is rare21,33–35 and usually occur in boys with choriocarcinoma because β-human chorionic gonadotropin (β-HCG) secondarily stimulates androgen secretion by the Leydig cells of the testes.
39.6 Diagnostic Studies
39.6.1 Neuroimaging
Computed Tomography
Computed tomography (CT) and magnetic resonance (MR) imaging are helpful for tumor detection. Associated abnormalities, such as hemorrhage and calcifications, may be better appreciated with CT. Calcifications often occur in the pineal gland. According to Zimmerman and Bilaniuk, the youngest subject with a normally calcified pineal gland was 6 1/2 years of age.36 The rates of pineal calcifications detected on CT scans were 8 to 10% from the ages of 8 to 14 years, 30% at the age of 15 years, and 40% at the age of 17 years. The presence of calcifications in children younger than 6 years, however, is abnormal and needs to be investigated for a neoplastic process.32
On CT scans, germinomas appear as soft tissue masses that are hyperdense relative to the surrounding brain tissue. Pineal calcifications of germinomas tend to be located centrally or displaced peripherally in the tumor mass, whereas calcifications of pineoblastomas, if present, are dispersed within the tumor (▶ Fig. 39.1). Teratomas tend to present as inhomogeneous hypodense multiple cystic lesions with variable contrast enhancement (▶ Fig. 39.2). After the infusion of intravenous contrast agent, germinomas tend to enhance homogeneously. NGGCTs tend to have similar homogeneous soft tissue density, and their calcification is alsosimilar to that of germinomas. Choriocarcinomas may have hemorrhagic foci (▶ Fig. 39.3). On the other hand, the appearance of teratomas is more distinct and often heterogeneous, with a variable degree of soft tissues, calcifications, and cyst formation. Pineoblastomas are relatively homogeneous and hyperdense on precontrast CT scans. After the infusion of contrast material, enhancement ranges from dense to little or none. Calcifications can occur either centrally or peripherally, but calcification is rare in pineoblastomas. There are no characteristic CT features to differentiate among pineal germ cell tumors other than teratomas and pineal parenchymal tumors.
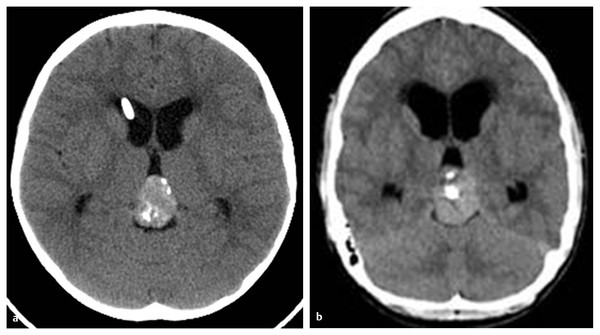
Fig. 39.1 Pineal calcification on precontrast computed tomographic scans. (a) Calcifications may be located centrally or peripherally in germinoma. (b) Calcifications may be dispersed in pineoblastoma.
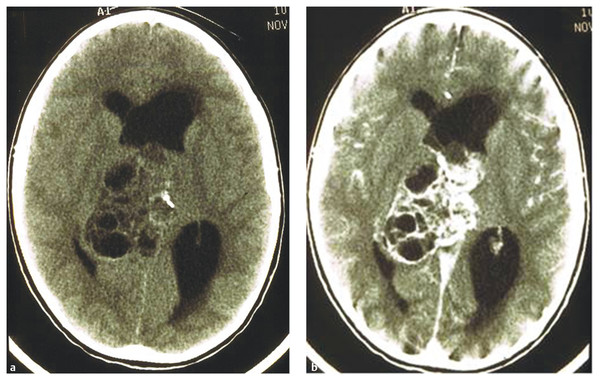
Fig. 39.2 Pineal calcification on precontrast computed tomographic scans. (a) Calcifications may be located centrally or peripherally in germinoma. (b) Calcifications may be dispersed in pineoblastoma.
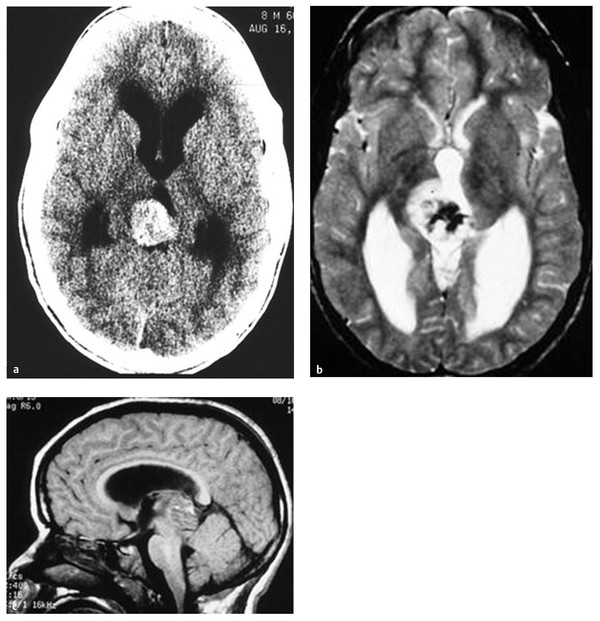
Fig. 39.3 (a) Hemorrhagic choriocarcinoma is shown on precontrast computed tomographic scan, (b) T2-weighted axial magnetic resonance (MR) image, and (c) T1-weighted sagittal MR image.
Benign astrocytomas are often hypodense relative to the surrounding brain tissues on precontrast CT scans. They primarily originate from the midbrain or the posterior thalamus. They may be cystic, but calcification is uncommon. Contrast enhancement is variable and often inhomogeneous. Dermoid and epidermoid tumors tend to be hypodense and enhance minimally after the infusion of contrast material. They may have density equal to that of CSF, and their appearance may mimic that of an arachnoid cyst or pineal cyst.
Magnetic Resonance Imaging
Teratomas are often heterogeneous, with the presence of cyst formation and fat (▶ Fig. 39.4). Germinomas show low signal intensity to isointensity on T1-weighted images. They show high signal intensity or are isointense on T2-weighted images.37 After the infusion of contrast material, germinomas show enhancement. Malignant NGGCTs may show variable signal intensity that is partly due to the presence of hemorrhage.38 Pineoblastomas are hypo- or isointense on T1-weighted images, and variable degrees of enhancement are noted with poorly defined margins.39 In general, the MR imaging signal characteristics are usually nonspecific,36,38 and correlation with the patient’s age, sex, and other associated factors needs to be analyzed for diagnostic purposes.39 Concurrent masses in both the anterior and posterior parts of the third ventricle strongly suggest germ cell tumor, germinoma in particular (▶ Fig. 39.5). Rates of occurrence of multiple germinoma lesions in the third ventricle may vary: 6.0 to 8.5%,8,21 15%,40 or 32 to 57% of cases.37,40,41 The frequency of this multiplicity has increased with the use of more sensitive neurodiagnostic methods. For instance, Sugiyama et al reported that the frequency of synchronous lesions in pineal and suprasellar germinomas increased from 4.2% before to 17.4% after the introduction of MR imaging.31 In my personal experience with 43 patients who had pineal germ cell tumors treated from 1988 to 2010, 12 (28%) showed synchronous anterior and posterior third ventricle lesions. Of these 12 patients, 10 presented with diabetes insipidus. When a patient with a pineal region tumor presents with diabetes insipidus, the pituitary stalk and the tuber cinereum should be carefully investigated in order to detect multiplicity of the lesions.
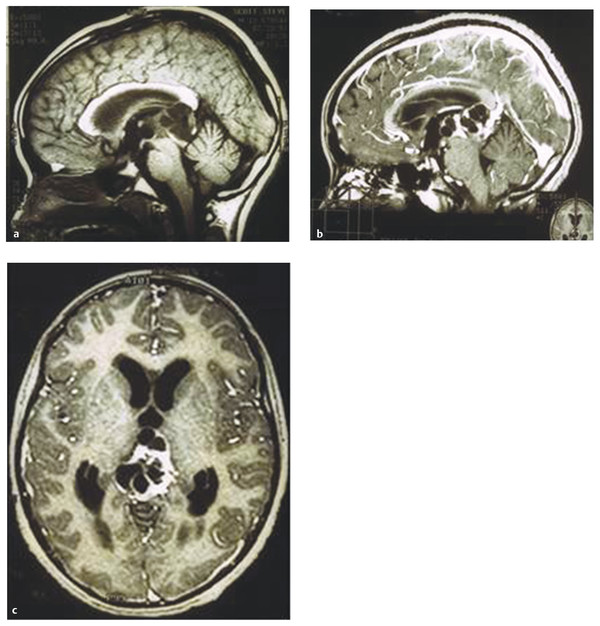
Fig. 39.4 (a) Mature teratoma is shown on precontrast sagittal magnetic resonance (MR) image. (b) Note inhomogeneous enhancement on postcontrast sagittal and (c) axial MR images.
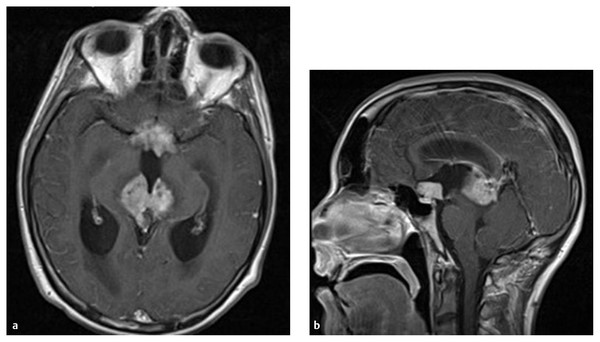
Fig. 39.5 (a) Axial and (b) sagittal magnetic resonance images after contrast infusion showing concurrent germ cell tumors with elevated serum alpha fetoprotein in the pineal and suprasellar locations.
Among patients with either unilateral or bilateral retinoblastomas, 3% have pineal parenchymal tumors (trilateral retinoblastoma). A history of retinal disease or evaluation with neuroimaging of the orbits enables the differentiation of pineoblastomas from other tumors in such a case.
Pineal region tumors are frequently associated with hydrocephalus. Pineal cysts, which are present in 2.4% of the normal population, are rarely symptomatic and usually remain stable in size.16 They have a contrast-enhancing cyst wall and subtle hyperintensity of the cyst contents. Mostly, they are single cysts, although occasionally a multicystic appearance is noted. Large cysts occasionally show radiographic evidence of tectal compression despite the absence of hydrocephalus. The occurrence of hydrocephalus is very rare. Fain et al reported that of 20 patients with symptomatic pineal cyst, 8 were noted to be hydrocephalic.17
A nonenhancing thick quadrigeminal plate that causes obstructive hydrocephalus rarely requires surgical biopsy. No histologic data are available for these lesions because of the lack of pathologic verification. Contrast enhancement within the lesion may vary during follow-up despite a stable size over years. Some may show an exophytic nature, projecting beyond the quadrigeminal plate, and/or contrast enhancement, mimicking a pineal tumor.
Once a pineal region tumor is diagnosed, MR imaging of the spine needs to be obtained for staging purposes. Some malignant tumors, pineoblastomas in particular, may present with diffuse dissemination in the subarachnoid space.
39.6.2 Laboratory Tests
Certain germ cell tumors manifest tumor markers that are identified in the serum and CSF. Identification is important not only for diagnostic purposes but also for monitoring responses to treatment and relapses.36 Alpha fetoprotein (AFP), a glycoprotein, is normally produced by the yolk sac and the fetal liver, but its production ceases by the time of birth. An AFP value of less than 5 ng/mL in the serum and CSF is considered to be normal.40 In the central nervous system, yolk sac tumors (endodermal sinus tumors) show the greatest production of AFP. Embryonal carcinomas and immature teratomas produce AFP to a lesser extent (< 1,000 ng/mL). β-HCG is a glycoprotein that is normally secreted by syncytiotrophoblastic giant cells in placental trophoblastic tissue. Therefore, the presence of β-HCG strongly indicates germ cell tumors. The normal value in the serum and CSF is less than 5 mIU/mL.40 Marked elevation of β-HCG above 2,000 mIU/mL is noted in choriocarcinomas, but mild elevations (< 770 mIU/mL) can occur in patients with germinomas and embryonal carcinomas. The biological half-life is about 5 days for AFP, whereas it is less than 24 hours for β-HCG. The titers of β-HCG and AFP reflect the number of cells secreting these glycoproteins; this information is useful for differentiating tumors that are predominantly choriocarcinoma or yolk sac tumor, but not confirmative for histologic diagnosis.21 Abnormal AFP and/or β-HCG levels in the serum or CSF are generally regarded to indicate NGGCTs.42
Malignant germ cell tumors show positivity for tumor markers, either β-HCG or AFP or both, in 39 to 70% of cases.21,37 The results of positivity for these tumor markers are variable; some report more frequent positivity for AFP43 and others vice versa.44 However, our observations (T. Tomita, unpublished data) and those of others45 indicate that the β-HCG value tends be higher in the CSF than in the serum.45 Thus, it is important to obtain the values of tumor markers from both serum and CSF.
Placental alkaline phosphatase (PLAP) was reported as a specific marker for primary intracranial germinomas.46 PLAP is distinguished from other common tissue alkaline phosphatases by its heat resistance and its inhibition by L-phenylalanine. PLAP levels in serum or CSF are measured with an enzyme-linked immunosorbent assay, but their sensitivity and specificity need further investigation. The CSF in five of nine patients with germinoma showed high levels of PLAP.47 However, reliable methods to measure PLAP in the CSF have not been available. The proto-oncogene c-KIT, which encodes tyrosine kinase for the stem cell factor, is expressed on the cell surface of germinoma. The concentration of the soluble form of the c-KIT receptor (s-KIT) is significantly high in the CSF among patients with germinoma.10,48 The melatonin level may be used as a tumor marker for pineal tumors. Melatonin secretion by the pineal gland follows a nyctohemeral rhythm. When the pineal gland is disturbed by invasive tumors such as germinoma or NGGCT, the melatonin rhythm is dramatically reduced. However, the diagnostic value of melatonin profiles is limited in clinical practice.49 Low serum levels of melatonin may be used as a marker for pineal tumors that destroy the pineal gland.50 High nighttime melatonin levels have been reported among patients with pineocytomas, whereas depressed melatonin secretion has been observed among patients with pineal cysts.51
39.6.3 Cerebrospinal Fluid Cytology
The CSF, whenever available, either by means of lumbar puncture or from the ventricle at shunt placement, third ventriculostomy, or external ventricular drainage, should be analyzed with cytologic studies together with tumor markers for pineal region tumors. The frequency of CSF dissemination among malignant germ cell tumors varies from 3%43,44 to 36 to 52%.52 On the other hand, pineoblastomas disseminate along the CSF pathway with higher frequencies of 50% at diagnosis and almost 100% at terminal stage.16,53
39.6.4 Biopsy
Histologic verification on the basis of tumor markers and/or biopsy leads to an appropriate selection of therapeutic mode. There are several modes of biopsy: stereotactic biopsy, neuroendoscopic biopsy, and open biopsy.
Stereotactic Biopsy
Most reports indicate that the risks of surgical intervention are minimal.54,55 However, it is a serious potential concern that stereotactic procedures may result in hemorrhagic complications because tumors originating from the pineal gland are surrounded by deep veins of the lesser galenic system. Also, some tumors, such as pineoblastomas and choriocarcinomas, are often extremely vascular. In my experience with 14 stereotactic biopsies of pineal region tumors, two patients (one with germinoma and one with pineoblastoma) experienced a serious hemorrhage into the tumor and ventricle. However, other authors have reported no major complications due to the stereotactic procedure.55
Neuroendoscopic Biopsy
Neuroendoscopic tumor biopsy from the third ventricle became popular after recent advances in endoscopic instrumentation. The use of an image-guided neuronavigational system aids in localization of the tumor at endoscopic biopsy. Endoscopic biopsy can be performed at the same time as endoscopic third ventriculostomy (ETV).56–58 The pineal region tumor, however, is often difficult to visualize in the posterior third ventricle when a standard trajectory is used for the third ventriculostomy. In order to overcome this difficulty, one may use a flexible scope or a 30-degree-angle rigid scope,57 or create another bur hole that is placed farther anteriorly.56 Occasionally, unexpected tumor seeding may be recognized though endoscopic observation that may not be apparent on neuroimaging evaluation. Hemostasis can be achieved with a bipolar cautery. However, controlling a major hemorrhage is very difficult because of the limited field for ventriculoscopic instrumentation compounded by poor visibility due to hemorrhagic CSF. Wong et al postulated that intratumoral hemorrhage during and after endoscopic procedures for pineal tumors may be due to loose tumor tissue, hypervascularity in the tumor, and abrupt reduction in the intracranial pressure to zero during endoscopic procedures.59 If significant hemorrhage occurs, the ventricular space should be irrigated until the returning fluid become clear. The patient may need temporary external ventricular drainage.
The tumor samples obtained with either a stereotactic procedure or ventriculoscopy are small. The pathologic diagnosis, however, can in most cases be determined from these small tumor samples. In cases of mixed malignant tumors, however, heterogeneous components may be overlooked, and a small sample obtained with these methods may not indicate the true nature of the tumors.
39.7 Treatment
39.7.1 Hydrocephalus
Hydrocephalus may be controlled with either tumor mass reduction or a CSF diversion procedure. The surgical or nonsurgical reduction of a tumor in the pineal region would open the occluded aqueduct of Sylvius. Certain germ cell tumors can be considerably reduced in size in a short time following radiation therapy (RT) or chemotherapy, and hydrocephalus is resolved without surgery. However, these nonsurgical methods of tumor mass reduction require at least several weeks. Steroid therapy may be used for hydrocephalus pending the efficacy of RT or chemotherapy. Germinomas that contain a significant number of T-lymphocytes may be reduced in volume following steroid therapy (▶ Fig. 39.6).
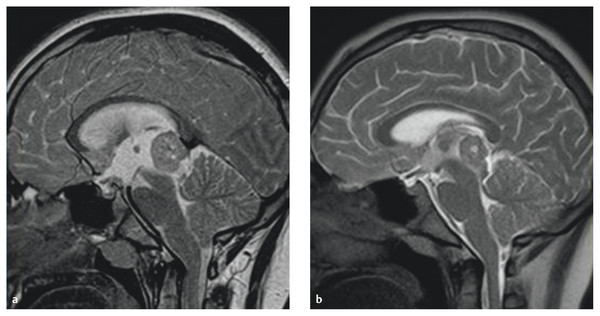
Fig. 39.6 (a) T2-weighted sagittal magnetic resonance image showing hydrocephalus secondary to pineal germinoma. (b) Following 3 days of treatment with dexamethasone, the tumor and hydrocephalus decreased in size.
A ventriculoperitoneal shunt was often used in the past. Placement of the shunt promptly improves the patient’s condition. Concerns of the shunt, however, are potential risks for peritoneal metastases through the shunt and shunt dependency.
Recent advances in neuroendoscopic technology have enabled us to perform ETV. Successful hydrocephalus control is reported in 80% of patients with pineal region tumors.58 The posterior third ventricle can be inspected and tumor biopsy can be performed at the same time as ETV. I routinely place an Ommaya reservoir connected to the ventriculostomy catheter during the ETV that is helpful for subsequent ventricular access.
39.7.2 Craniotomy
In 1921, Dandy initially described a parietal interhemispheric transcallosal approach.60 Later, Horrax in 193761 and subsequently Poppen in 196662 developed an occipital transtentorial approach through a supratentorial craniotomy. The infratentorial route is primarily approached along the superior surface of the cerebellum and the inferior surface of the tentorium. This infratentorial supracerebellar approach was first described by Krause in 192663 and was popularized by Stein.64
The current success in pineal tumor resections has been made possible by the surgical microscope, microsurgical instrumentation, established surgical approaches, and advanced neuroimaging studies. Intraoperative imaging guidance is also helpful. Approaches are either supratentorial or infratentorial.65 The supratentorial routes include an occipital transtentorial approach, posterior interhemispheric approach, posterior interhemispheric transcallosal approach, anterior interhemispheric transcallosal approach, and lateral transventricular approach.
Each approach has advantages and disadvantages, and the approach should be chosen on the basis of the anatomical information provided by neurodiagnostic images, along with the surgeon’s familiarity with and confidence in using the approach. The tumor location and extent, the deep venous system, and the surrounding neural structures need to be considered when the surgical approach is chosen. Other factors are the patient’s age, presence or absence of hydrocephalus, shape of the head, and purpose of the surgery (biopsy vs. total resection). I personally prefer the supratentorial approach because pineal region tumors in children tend to be large and extend in various directions, and also because the posterior fossa space in infants and children is relatively small. The supratentorial approach provides a wide range of trajectory angles through a large interhemispheric avenue.
Supratentorial Approach
Occipital Transtentorial Approach
The prone position is used routinely, although some prefer a sitting position. I personally prefer to place all pediatric patients in a prone position with the head turned slightly (about 15 degrees) away from the surgical side if the patient is old enough to receive head pins for a head holder. This position is designed to let gravity cause the occipital lobe fall away from the falx, without the need for forcible brain retraction. Some choose a recumbent position for that reason.66 If the patient is very young, when a pin fixation device is not applicable, the head is rested on a well-padded horseshoe head holder; the head is not turned. Brain retraction is controlled by means of intraoperative ventricular drainage for the interhemispheric approach. Retraction of the occipital and parietal lobes is more restricted when the ventricle is small or slitlike. One should avoid forcible retraction of the occipital lobe. The brain needs to be relaxed with the administration of mannitol, or sometimes by externalizing an existing shunt intraoperatively.
The occipital transtentorial approach provides a wide surgical entry for access to the pineal region. One can select from a wide range of angles of entry through a craniotomy that extends from the inion to the entry site of the last cortical vein into the superior sagittal sinus, which is usually 3 to 5 cm rostral to the lambda. Exposure of the lateral sinus is not necessary because the approach is not suboccipital (lifting the occipital lobe) but through the interhemispheric fissure along the falx. Through a hockey stick skin incision (▶ Fig. 39.7), a craniotomy is usually placed that is approximately 10 cm in length and 5 cm in width and crosses over the superior sagittal sinus to the opposite side (▶ Fig. 39.8). At the entry of the interhemispheric fissure, there usually are no cortical veins posterior to the posterior parietal region. In my experience with 68 posterior interhemispheric approaches, only one patient had a significant draining vein from the occipital pole. However, Davidson et al stated that 7 of 27 patients needed bridging vein sacrifice through this approach.66
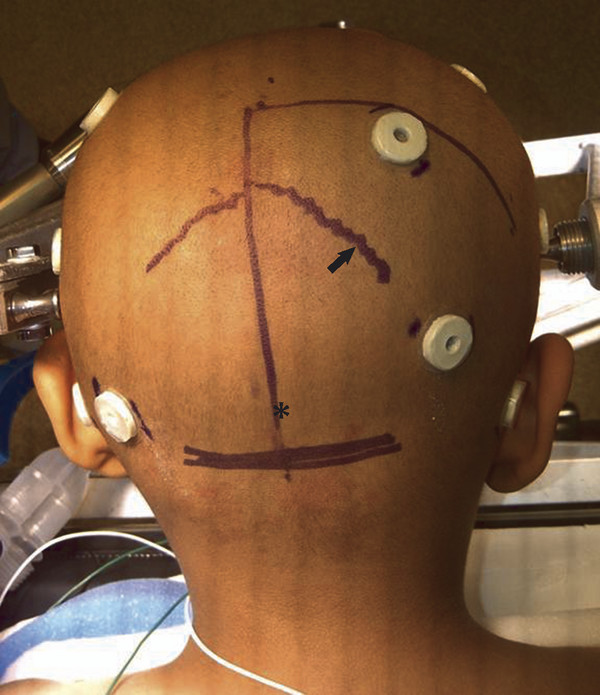
Fig. 39.7 Hockey stick skin incision for a right-sided occipital craniotomy and an occipital transtentorial approach. Note the right-sided lambdoid suture (arrow) and the inion (asterisk).









