29 Postoperative Care and Complications Management • Major complication rates in skull base surgery have been reported in the range of 10 to 50%.1,2 • Emergency case • Requirement of blood transfusion before surgery • Presence of preexisting neurologic symptoms (altered mental status, paraplegia/paraparesis or quadriplegia/quadriparesis, prior stroke) • Complex surgical approach and prolonged anesthetic treatment • Preexisting comorbidities, especially cardiovascular and pulmonary diseases • Alcohol use • Chronic steroid use Pearl A team-oriented approach involving surgeons, anesthesiologists/intensivists, and other specialized health care providers is recommended to manage these challenging patients. The overall goal of this approach is prevention, early identification, and correct management of complications that can arise as a result of surgery. • The importance of patient handover (Table 29.1): Appropriate care and monitoring of the postoperative neurosurgical patient requires accurate knowledge of • Admission considerations: Immediate postoperative care and monitoring of patients after skull base surgery is traditionally provided in an intensive care unit (ICU) or in a high-dependency unit with advanced monitoring capability. However, in selected patients, ICU admission may not be necessary.5 The required level and duration of monitoring after skull base surgery is determined by the following: 1. Preoperative clinical status and comorbidities 2. Characteristics of the surgical procedure 3. Intraoperative events 4. Specific needs in the immediate postoperative period Pearl Most of the major complications develop intraoperatively or in the immediate postoperative phase, with a very small number of postoperative neurosurgical patients requiring intensive monitoring beyond the first 6 to 24 hours, according to specific risk profiles. The majority of postoperative hematomas present with clinical signs of neurologic deterioration within 6 hours of surgery; therefore, an intensive but short postoperative monitoring period may be a cost-effective strategy in most neurosurgical patients.5,6 • Monitoring strategies: The goal of postoperative monitoring is to identify as soon as possible patients who are deteriorating after surgery. Any delay in identifying and treating these patients may severely affect the outcome. • Systemic monitoring: Cardiovascular and respiratory monitoring is essential in patients undergoing skull base surgery (Table 29.1). • Neuromonitoring (Table 29.1): Routine assessment of level of consciousness and pupillary reactivity, and a neurologic examination focused on detection and monitoring of focal deficits are the cornerstones of early detection of postoperative neurologic deterioration. Anything that interferes with the ability to perform this assessment should be avoided when possible. The assessment should be repeated at regular intervals (ideally every hour initially, if possible) and any time there are signs of change in clinical status (e.g., changes in respiratory pattern, blood pressure, increased intracranial pressure (ICP), if monitored). Pearl Both systemic monitoring and neuromonitoring strategies are essential. Early reestablishment of consciousness is ideal, to enable clinicians to administer a neurologic exam. Table 29.1 Postoperative Considerations
 Infratentorial meningioma and craniopharyngioma are associated with higher rates of complications (medical and neurologic) than convexity lesions.3
Infratentorial meningioma and craniopharyngioma are associated with higher rates of complications (medical and neurologic) than convexity lesions.3
 For management of diabetes insipidus (DI), syndrome of inappropriate antidiuretic hormone (SIADH), and postoperative endocrine management, see Chapter 9.
For management of diabetes insipidus (DI), syndrome of inappropriate antidiuretic hormone (SIADH), and postoperative endocrine management, see Chapter 9.
 Predictors of Complications for All Neurosurgical Cases4
Predictors of Complications for All Neurosurgical Cases4
 Admission Considerations and Monitoring Strategies
Admission Considerations and Monitoring Strategies
 preoperative clinical status and comorbidities
preoperative clinical status and comorbidities
 intraoperative procedure
intraoperative procedure
 problems that may have occurred during surgery
problems that may have occurred during surgery
 anesthesia.
anesthesia.
STEP 1: The Patient Handover | ||
1.A. Preoperative Data | 1.B. Intraoperative Data | 1.C. Immediate Postoperative Course |
1.A.1. Neurosurgical data • Neurosurgical diagnosis • Neuroimaging results • Presurgical neurologic status (level of consciousness, presence of neurologic deficits, history of seizures) • Previous neurosurgical interventions 1.A.2. Preoperative clinical status • Medical comorbidities (especially cardiovascular, respiratory, and neurohormonal diseases) • Preoperative medications • Relevant laboratory test results • Allergy • Mechanical DVT prophylaxis | 1.B.1. Neurosurgery • Surgical goals and concerns • Patient surgical position • Surgical approach/technique • Duration of surgery • Intraoperative findings • Surgical issues and complications (e.g., brain swelling, brain ischemia, difficult hemostasis/intraoperative hemorrhage, temporary/permanent vascular occlusion, leaks, venous air embolism, nerve injury, rhabdomyolysis, etc.) 1.B.2. Anesthesia • Airway management (technique used for intubation, level of difficulty, trauma to the airway/dental injury) • Type of anesthesia and analgesia used • Intravascular accesses available • Blood loss, transfusions, and fluid balance • Intraoperative laboratory values • Intraoperative complications/secondary brain insults (e.g., hypoxemia, hypercapnia, hypotension, hypertension, intraoperative seizures, etc.) • Hypothermia/hyperthermia • Neuropsychiatric complications (e.g., emergence delirium, agitation, somnolence, visual disturbance, etc.) | • Postoperative pain • Postoperative nausea and vomiting • Seizures • Respiratory complications (e.g., residual neuromuscular blockade, airway obstruction, laryngospasm, airway edema, compromised oxygen exchange) • Cardiovascular complications (e.g., hypotension, hypertension, arrhythmias) |
2.A. General postoperative monitoring strategy • Cardiovascular monitoring: continuous ECG and blood pressure (invasive or noninvasive)a • In specific situations may consider central venous pressureb and other advanced hemodynamic monitoring strategies (e.g., pulmonary artery catheter, continuous arterial pulse-contours analysis devices, transpulmonary thermodilution technique)c • Respiratory monitoring: pulse oximetry (SpO2) and respiratory rate monitoring. Consider end-tidal carbon dioxide (ETCO2).d • Fluid balance assessment • Temperature: peripheral temperature and/or core temperature • Laboratory examination: blood gases, hematology (WBC, PLTs, Hgb), electrolytes, glucose, coagulation (INR, PTT). If indicated, consider lactate, cardiac markers (troponin) and CK (if rhabdomyolysis is suspected). In case of pituitary surgery, consider cortisol, thyroid hormones, LH, FSH, bioavailable testosterone, prolactin, free T4, and IGF-1. | ||
2.B. Neuromonitoring strategy • Neurologic examination: level of consciousness (e.g., Glasgow Coma Scale, or FOUR score), pupillary reactivity, and comprehensive or focused neurologic examination (detection and monitoring of focal deficits, cranial nerves dysfunctions) • Neurophysiology-based assessment (if indicated)
| ||
2.C. Instructions for postoperative care • General postoperative care (e.g., activity level allowed, position of the head of the bed, nutrition, goals and plan for airway management, if patient still intubated) • Postoperative medications (e.g., need for antiseizure medications, antibiotics, steroids, analgesia, sedation, hyperosmolar agents, stress ulcer prophylaxis) • Prevention of venous thromboembolism: mechanical prophylaxis (preferably with sequential intermittent pneumatic compression devices) versus pharmacological prophylaxis • Instructions for management of drains and tubes (including EVDs), stitches, and monitors • Instructions for postoperative follow-up imaging (e.g., CT or MRI, X-rays), if indicated/required | ||
Abbreviations: CK, creatine kinase; CT, computed tomography; DVT, deep vein thrombosis; ECG, electrocardiogram; EVD, external ventricular drain; FSH, follicle-stimulating hormone; Hgb, hemoglobin; IGF-1, insulin-like growth factor-1; INR, international normalized ratio; LH, luteinizing hormone; MRI, magnetic resonance imaging; PLT, platelets; PTT, partial thromboplastin time; T4, thyroxine; WBC, white blood count.
Source: Data from references 12, 14–17, and 78.
bThe use of central venous pressure monitoring to assess fluid status and fluid responsiveness, despite still being widely diffused, has been recently challenged. A recent meta-analysis looking at its utility to predict fluid responsiveness concluded that there are no data to support this practice and recommends to abandon this approach in fluid resuscitation.77
 Glasgow Coma Scale (GCS)7: The best motor score response is considered the most important parameter.
Glasgow Coma Scale (GCS)7: The best motor score response is considered the most important parameter.
 The FOUR (Full Outline of UnResponsiveness) score incorporates assessment of brainstem reflexes and ventilation pattern, and overcomes the failure to assess the verbal score in intubated patients8 (Table 29.2). The FOUR score has been shown to have a better ability to predict outcome than the GCS, but is more difficult to administer than the GCS and has not gained wide implementation.
The FOUR (Full Outline of UnResponsiveness) score incorporates assessment of brainstem reflexes and ventilation pattern, and overcomes the failure to assess the verbal score in intubated patients8 (Table 29.2). The FOUR score has been shown to have a better ability to predict outcome than the GCS, but is more difficult to administer than the GCS and has not gained wide implementation.
Table 29.2 Full Outline of UnResponsiveness (FOUR) Score
Eye response | 4 = Eyelids open or opened, tracking, or blinking to command 3 = Eyelids open but not tracking 2 = Eyelids closed but open to loud voice 1 = Eyelids closed but open to pain 0 = Eyelids remain closed with pain |
Motor response | 4 = Thumbs-up, fist, or peace sign 3 = Localizing to pain 2 = Flexion response to pain 1 = Extension response to pain 0 = No response to pain or generalized myoclonus status |
Brainstem reflexes | 4 = Pupil and corneal reflexes present 3 = One pupil wide and fixed 2 = Pupil or corneal reflexes absent 1 = Pupil and corneal reflexes absent 0 = Absent pupil, corneal, and cough reflex |
Respiration | 4 = Not intubated, regular breathing pattern 3 = Not intubated, Cheyne-Stokes breathing pattern 2 = Not intubated, irregular breathing 1 = Intubated, breathes above ventilator rate 0 = Breathes at ventilator rate or apnea |
Source: From Wijdicks EFM, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: The FOUR score. Ann Neurol 2005;58:585–593. Copyright © 2005 American Neurological Association. Reprinted with permission.
 Pupillary reactivity: Changes in the pupillary size and reactivity can provide important information on neurologic deterioration, presence of increased ICP, or herniation syndromes.9 The automated pupillometer provides more accurate and sensitive measurements, although its clinical usefulness and reliability are still controversial.10
Pupillary reactivity: Changes in the pupillary size and reactivity can provide important information on neurologic deterioration, presence of increased ICP, or herniation syndromes.9 The automated pupillometer provides more accurate and sensitive measurements, although its clinical usefulness and reliability are still controversial.10
 Neurologic examination: It is necessary to evaluate (1) persistence/worsening of previous deficits, (2) improvement of previous deficits after surgery, and (3) appearance of new neurologic findings.
Neurologic examination: It is necessary to evaluate (1) persistence/worsening of previous deficits, (2) improvement of previous deficits after surgery, and (3) appearance of new neurologic findings.
Pearl
Challenge: differentiation between normal postoperative findings (often related to residual effects of general anesthesia) and symptoms/signs indicative of postoperative complications (e.g., intracranial hematoma, brain swelling).
 Common “normal” findings: transient headache; upturned Babinski reflex up to 2 hours postsurgery; depressed pupillary response, unilateral pupillary dilatation in awake patients, or eccentric pupil; dysarthria; asterixis; mild exacerbation of previous hemiparesis (normally lasting no more than 2 hours); unsustained clonus; and shivering unrelated to body temperature.11–13
Common “normal” findings: transient headache; upturned Babinski reflex up to 2 hours postsurgery; depressed pupillary response, unilateral pupillary dilatation in awake patients, or eccentric pupil; dysarthria; asterixis; mild exacerbation of previous hemiparesis (normally lasting no more than 2 hours); unsustained clonus; and shivering unrelated to body temperature.11–13
 Concerning findings suggestive of postoperative complications: rapidly increasing headache (especially with associated vomiting), progressive drowsiness, seizures, new evolving hemiparesis, new or worsening paresthesias, vertigo, facial paresis, and pupillary changes in a fully awake patient.12
Concerning findings suggestive of postoperative complications: rapidly increasing headache (especially with associated vomiting), progressive drowsiness, seizures, new evolving hemiparesis, new or worsening paresthesias, vertigo, facial paresis, and pupillary changes in a fully awake patient.12
Pearl
Persistent weakness or bilateral ophthalmoplegia can be found after administration of nondepolarizing neuromuscular blockers. However, presence of ophthalmoplegia should always be investigated carefully before being attributed to neuromuscular blocking agents or emergence from anesthesia, given its association with basilar artery thrombosis.12
 Neurophysiology-based assessment, consisting of advanced intracranial monitoring (e.g., ICP, cerebral blood flow and brain oxygen delivery, bio-electrical activity, cerebral metabolism), is recommended in patients with poor baseline neurologic status or requiring continuous sedation for concurrent nonneurologic complications. This form of assessment is rarely indicated for the elective, uncomplicated, skull base patient.14–16
Neurophysiology-based assessment, consisting of advanced intracranial monitoring (e.g., ICP, cerebral blood flow and brain oxygen delivery, bio-electrical activity, cerebral metabolism), is recommended in patients with poor baseline neurologic status or requiring continuous sedation for concurrent nonneurologic complications. This form of assessment is rarely indicated for the elective, uncomplicated, skull base patient.14–16
In case of significant changes in the neurologic status:
1. Immediate reassessment of patient clinical stability (ABC: airway, breathing, and circulation). If severely depressed level of consciousness (GCS ≤ 8, rapidly deteriorating level of consciousness, and uncontrolled seizures), early tracheal intubation is essential to protect the patient’s airway (increased risk of aspiration, hypoxemia, and hypercarbia). Close monitoring of gas exchange should be undertaken with the following goals: normal oxygenation (oxygen saturation > 94%, while avoiding hyperoxia), and normocarbia (partial pressure of carbon dioxide in arterial gas [PaCO2] 35–45 mmHg). If signs of brain herniation are present or in the setting of increased ICP, hyperventilation should be instituted to acutely decrease ICP (goal PaCO2: 28–32 mmHg). However, hyperventilation should never be instituted as a definitive treatment (risk of brain ischemia and rebound elevation of ICP), and normocarbia should be rapidly reinstituted as soon as other treatments to control ICP are in place.17
2. Urgent brain computed tomography (CT) scan ± intravenous contrast.
 Blood Pressure Monitoring
Blood Pressure Monitoring
Postoperative hypertension is relatively common in neurosurgical patients, and it has been associated with a higher incidence of intracerebral hemorrhage, ischemic stroke, worsening edema, and myocardial infarction.18,19 Acute elevation of blood pressure can cause intracranial complications. Alternatively, it can be a physiological response to maintain cerebral perfusion pressure in the presence of intracranial injury. Therefore, careful blood pressure monitoring and its tight control are among the most important strategies in preventing complications in postoperative neurosurgical patients. Postoperative acute hypertension can be attributed to different causes such as under-controlled pain, anxiety, and preexisting arterial hypertension, or it can be a sentinel of a cerebrovascular event (e.g., intracerebral bleeding at operative site, subdural hematoma, or epidural bleeding).
Management
• No absolute threshold can be recommended (the level to which elevated blood pressure should be lowered depends on several factors).
• The first step in the management of postoperative acute hypertension is the identification of the underlying problem (evaluate the patient for neurologic status changes, inadequate analgesia, bladder distention, shivering, etc.).
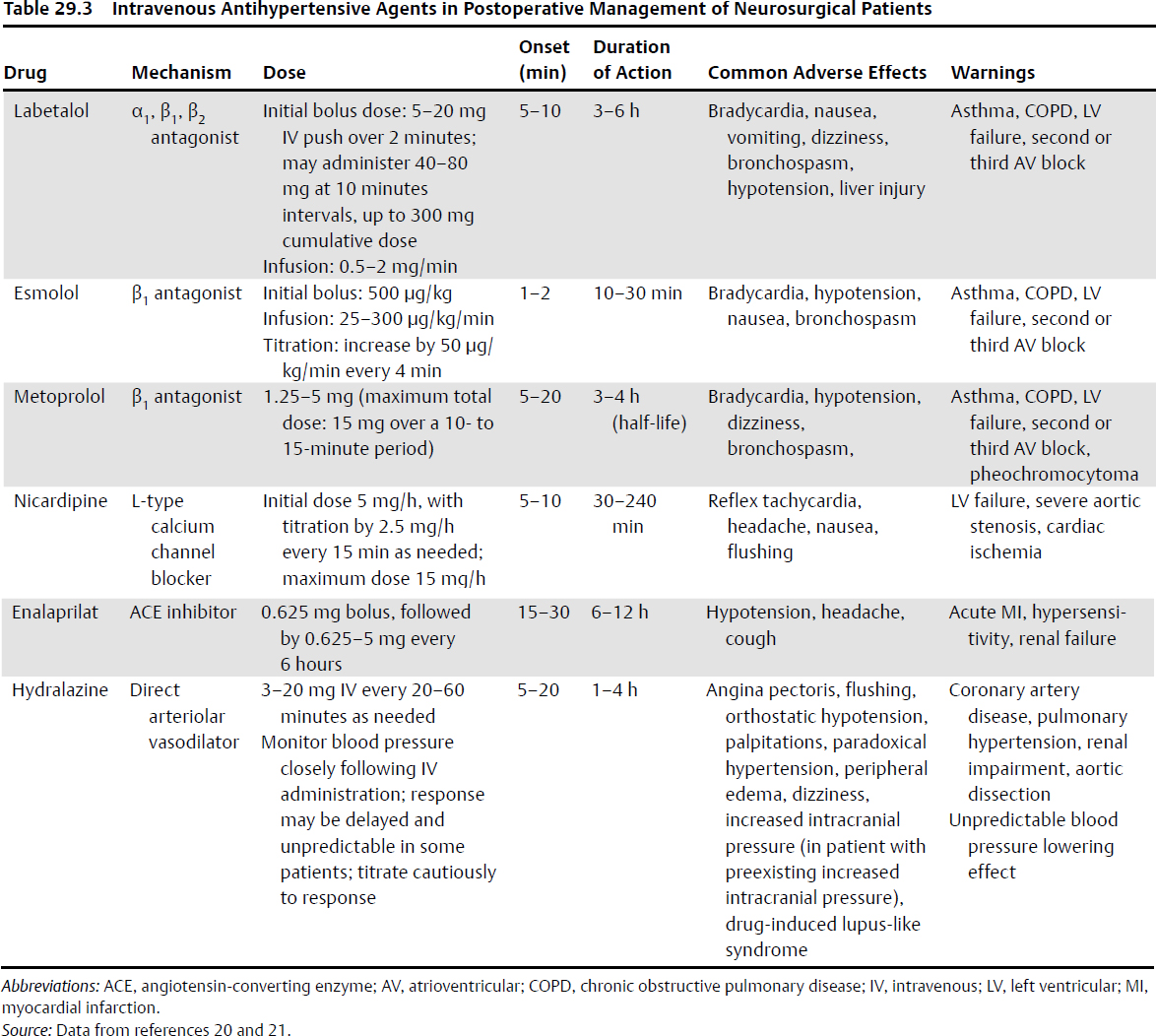
• If, after neurologic assessment, sufficient pain control, and exclusion of other reversible causes, the decision is made to control blood pressure with antihypertensive drugs, a short-acting, titratable, intravenous agent should be administered to achieve the target blood pressure quickly, while minimizing the risk of cerebral hypoperfusion. Intravenous β-adrenergic antagonists (labetalol, metoprolol, esmolol) are an acceptable choice for treatment if there are no contraindications. With refractory hypertension or marked bradycardia, angiotensin-converting enzyme (ACE) inhibitors (e.g., enalapril) and vasodilators (e.g., hydralazine or calcium channel blockers) can be used. Nicardipine is considered the safest calcium channel blocker. Concerns regarding elevated ICP accompany the use of calcium channel blockers. Sodium nitroprusside and nitroglycerin increase ICP and lower cerebral blood flow, and should therefore be avoided in patients with reduced intracerebral compliance (Table 29.3).20,21 In patients with chronic hypertension, reinstitute the patient’s previous oral antihypertensive medications early.12
Postoperative Hypotension
Postoperative hypotension following elective neurosurgery is uncommon (2–5%), and it has been associated with poorer outcome. Immediate resuscitation with fluid and possibly vasoactive agents should be instituted to avoid cerebral hypoperfusion. Transfusion therapy should also be considered. Causes of shock should be rapidly investigated and treated (hypovolemic, neurogenic, distributive, cardiogenic, and obstructive shock).
 Prevention of Venous Thromboembolism
Prevention of Venous Thromboembolism
Neurosurgical patients are at high risk of developing venous thromboembolism (VTE).
• Pulmonary embolism has been reported in up to 5% of neurosurgical patients (mortality between 9% and 50%).22
• Estimated deep vein thrombosis (DVT) incidence ranges from 19 to 43% when standardized screening protocols are implemented.23 In neurosurgical oncology, DVT has been shown to be the most common adverse event.2 Meningiomas are associated with the highest postoperative rate of VTE events (the risk is three times higher than in patients with other brain tumors), especially skull base meningiomas.24,25
 Factors associated with increased risk of VTE in neurosurgical patients include cancer; advanced age; longer duration of surgery; prolonged immobilization (pre-, intra-, and postoperative); and the release of thromboplastin secondary to brain surgery.
Factors associated with increased risk of VTE in neurosurgical patients include cancer; advanced age; longer duration of surgery; prolonged immobilization (pre-, intra-, and postoperative); and the release of thromboplastin secondary to brain surgery.
Longer duration of surgery, longer postoperative immobilization, and disease-specific morbidity (involvement of cranial nerves, pituitary stalk, or vascular structures) are contributing factors to the higher incidence of VTE events in skull base meningiomas. However, pharmacological VTE prophylaxis is frequently debated in neurosurgery because of the potential increased risk of intracranial bleeding (estimated baseline risk of intracranial hemorrhage [ICH] postcraniotomy: 1.1%, depending on patient- and procedure-specific factors26).
Management
Postoperative management of VTE prophylaxis is a balancing act between the risk of thrombotic events and the risk of intracerebral bleeding. Based on current evidence and available strategies,23,27 the 2012 American College of Chest Physicians (ACCP) Guidelines28 suggest risk stratification, with an individualized approach based on the risk of VTE (Table 29.4). In selected patients with specific risk of ICH (e.g., tumor type, intraoperative findings, and course of neurosurgical procedure), an even more individualized approach should be considered. As a general measure applicable to every group of patients, early mobilization of patients, if appropriate, should be instituted.
High Risk for Venous Thromboembolism Patients (Elective Craniotomy for Nonmalignant Disease)
Mechanical Techniques
The ACCP guidelines28 suggest the use of mechanical prophylaxis, preferably with sequential intermittent pneumatic compression (IPC) devices (rather than no prophylaxis, grade 2C, or pharmacological VTE prophylaxis, also grade 2C).
Table 29.4 Venous Thromboembolism (VTE) Risk Stratification
Type of Patient | Risk Profile |
Patient undergoing craniotomy for nonmalignant disease | High risk for VTE (~ 5%) |
Patient undergoing craniotomy for malignant disease | Very high risk for VTE (≥ 10%) |
Patient with traumatic brain injury | Very high risk for VTE (≥ 8–10%) |
Source: Data from Gould MK, Garcia DA, Wren SM, et al; American College of Chest Physicians. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2, Suppl):e227S–e277S.
• IPC devices are favored over graduated compression stockings due to the increased risk of skin complications with elastic stockings.
• In cases of preexisting DVT or baseline immobility (potential presence of undiagnosed DVT), IPCs should not be used without ultrasonographic confirmation of the absence of DVT.
• IPC devices can be discontinued when the patient is able to ambulate at least 3 to 4 hours per day.
Pharmacological VTE prophylaxis with unfractionated heparin (UFH) or low molecular weight heparins (LMWH) at prophylactic doses is not recommended in high-risk neurosurgical patients
• In high-risk patients, the ACCP guidelines28 consider pharmacological prophylaxis with LMWH more harmful than beneficial:
 It is associated with a possible increase in the risk of death from any cause.
It is associated with a possible increase in the risk of death from any cause.
 LMWH may prevent between eight and 36 VTE events/1,000 patients, but at a cost of four to 22 additional ICH events/1,000 patients.
LMWH may prevent between eight and 36 VTE events/1,000 patients, but at a cost of four to 22 additional ICH events/1,000 patients.
• Low-dose UFH seems to have a more favorable trade-off, but the evidence is lacking.
• Therefore, the guidelines do not recommend UFH in high-risk neurosurgical patients.
Patients with Very High Risk for VTE (Elective Craniotomy for Malignant Disease)
• The use of IPC devices, low-dose UFH, or (possibly) LMWH seems to be associated with better outcome in very high-risk craniotomy patients as compared with no prophylaxis. However, when comparing LMWH with IPC devices, despite being associated with more nonfatal VTE events (6–26 more events/1,000 patients treated), IPC devices entail fewer episodes of nonfatal ICH (4–22 less events/1,000 patients treated)
• Adding pharmacological prophylaxis seems to prevent an additional 23 VTE events/1,000 patients treated, at the expense of 11 more ICH events/1,000 patients (considering 4.0% as the risk of symptomatic VTE in patients receiving IPC devices alone).
 The current ACCP Guidelines28 suggest a combination strategy in very high-risk patients, with the addition of pharmacological prophylaxis to mechanical prophylaxis once adequate hemostasis is established and the risk of bleeding decreases (grade 2C).
The current ACCP Guidelines28 suggest a combination strategy in very high-risk patients, with the addition of pharmacological prophylaxis to mechanical prophylaxis once adequate hemostasis is established and the risk of bleeding decreases (grade 2C).
 Surgical consideration of risk of bleeding is essential. Patients with significant pial invasion and dissection, large tumors, large exposures, and residual vascular lesions may be at higher risk of postoperative hemorrhage.
Surgical consideration of risk of bleeding is essential. Patients with significant pial invasion and dissection, large tumors, large exposures, and residual vascular lesions may be at higher risk of postoperative hemorrhage.
 Most postoperative ICHs occur in the first 48 hours after craniotomy, whereas the vast majority of VTE events occurs in the first week or later. Therefore, the most favorable time for adding LMWH or low-dose UFH to IPC devices seems to be 24 to 48 hours after craniotomy, as long as adequate hemostasis has been achieved and the risk of bleeding is judged as not being excessively high.
Most postoperative ICHs occur in the first 48 hours after craniotomy, whereas the vast majority of VTE events occurs in the first week or later. Therefore, the most favorable time for adding LMWH or low-dose UFH to IPC devices seems to be 24 to 48 hours after craniotomy, as long as adequate hemostasis has been achieved and the risk of bleeding is judged as not being excessively high.
 A study shows that early use of subcutaneous low-dose UFH at either 24 or 48 hours was associated with a 43% reduction of lower extremity DVT without an increased incidence of ICHs.29
A study shows that early use of subcutaneous low-dose UFH at either 24 or 48 hours was associated with a 43% reduction of lower extremity DVT without an increased incidence of ICHs.29
Patients with Traumatic Brain Injury (Very High Risk for VTE)
• The current ACCP Guidelines28 suggest the use, as soon as possible, of mechanical prophylaxis (preferably with IPC) rather than no prophylaxis when not contraindicated by lower-extremity injury (grade 2C).
• If the risk of bleeding diminishes, most authors suggest adding pharmacological prophylaxis, ideally at 24 hours (but definitively within 48 hours).
• Inferior vena cava filters are not suggested for primary VTE prevention (grade 2C).
 Stress Ulcer Prophylaxis
Stress Ulcer Prophylaxis
Critically ill patients are at risk of stress-related gastrointestinal mucosal damage, which can progress to ulceration and overt gastrointestinal (GI) bleeding. In the absence of adequate pharmacological prophylaxis, GI bleeding has been reported in 5 to 25% of critically ill patients, with an estimated incidence of clinically important GI bleeding varying between 0.1% and 4% in the most recent studies.30–33
Management
• Most neurosurgical postoperative patients, even if receiving corticosteroids, do not have high-risk profiles (Table 29.5) for GI bleeding and therefore should not receive stress ulcer prophylaxis.
• For patients at high-risk for GI complications, stress ulcer prophylaxis is currently considered the standard of care. The American Society of Health System Pharmacists34 recommends stress ulcer prophylaxis for patients with at least one major risk factor or at least two other risk factors (Table 29.5).
Table 29.5 Risk Factors for Overt Gastrointestinal (GI) Bleeding
Major Risk Factors | Other Risk Factors* |
• Mechanical ventilation ≥ 48 hours (odds ratio [OR] 15.6) • Coagulopathy (OR 4.3) | • Acute hepatic failure • Acute kidney injury • Nasogastric tube placement ≥ 5 days • History of alcohol abuse • Chronic renal failure • Positive for Helicobacter pylori • Severe head or spinal cord injury • Thermal injury (> 35% body surface area) • High-dose corticosteroids |
Source: Data from references 30, 33, and 78.
*These factors are less strongly associated with significant GI bleeding.
• Suggested drugs are histamine-2 receptor antagonists (H2RAs) or proton pump inhibitors (PPIs). PPIs can significantly lower the risk of clinically important and overt gastrointestinal bleeding, without influencing ICU mortality or length of ICU stay (Table 29.6). Optimal resuscitation and early enteral nutrition seem to be key factors in further preventing stress ulcer development.
There are some possible harms from pharmacological stress ulcer prophyl-axis agents: H2RAs and PPIs have been associated with adverse drug events and increased medication costs, and they seem to enhance susceptibility to nosocomial pneumonia and Clostridium difficile infection.33
 Anemia
Anemia
Bleeding and anemia are among the most common complications following neurosurgical procedures,3 and may require red blood cell (RBC) transfusion.
• Current evidence supports a restrictive RBC transfusion strategy (hemoglobin [Hb] threshold: ~ 70 g/L) in general critical care patients with normal brain function.35,36 Moreover, in healthy subjects, brain hypoxia and dysfunction have been shown to manifest only when Hb is less than 60 to 70 g/L.
• Some physiological studies and observational data suggest that acute brain injury is exacerbated when Hb falls below 100 g/L.37–39 In contrast, data on the effect of anemia in stable postoperative neurosurgical patients are very limited.40
Table 29.6 Pharmacological and Nonpharmacological Stress Ulcer Prophylaxis Agents
• RBC transfusion has been shown to be an independent risk factor for adverse outcomes and complications in both general critical care35,41 and neurocritical care patients.42,43
Pearl
In brain-injured patients at high risk of secondary brain injury, the exact level at which anemia threatens tissue oxygenation and at which RBC transfusion is more beneficial than harmful is currently unknown (no level I evidence available).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Intracranial pressure (ICP) and cerebral perfusion pressure (CPP) monitoring
Intracranial pressure (ICP) and cerebral perfusion pressure (CPP) monitoring Cerebral blood flow and oxygen delivery monitoring: cerebral blood flow devices, transcranial Doppler, jugular venous oximetry (SjO2), near-infrared spectroscopy (NIRS), brain tissue oxygen partial pressure (PbtO2)
Cerebral blood flow and oxygen delivery monitoring: cerebral blood flow devices, transcranial Doppler, jugular venous oximetry (SjO2), near-infrared spectroscopy (NIRS), brain tissue oxygen partial pressure (PbtO2) Bioelectrical activity monitoring: electroencephalogram (EEG), evoked potentials
Bioelectrical activity monitoring: electroencephalogram (EEG), evoked potentials Cerebral metabolism monitoring: cerebral microdialysis
Cerebral metabolism monitoring: cerebral microdialysis Biomarkers
Biomarkers Level of consciousness
Level of consciousness