Pregabalin
Gregory K. Bergey
Introduction
Pregabalin is the most recent antiepileptic drug (AED) introduced in the United States; it received approval as adjunctive therapy for partial seizures in 2005. Pregabalin has also been approved for treatment of neuropathic pain associated with diabetic neuropathy55,57 and postherpetic neuralgia.22,59 Several controlled trials have been done with pregabalin in generalized anxiety disorder (GAD), and approval for GAD exists in other countries, but not in the United States.44,45,48,49,52 Pregabalin is a congener of gabapentin (Chapter 149), an AED approved in 1994.60 Although pregabalin and gabapentin share certain features, important distinctions also exist. Because these two compounds are chemically related, and there is considerable experience with gabapentin, this chapter will specifically include a discussion of the similarities and differences between the two drugs, where they are known. Although comments regarding efficacy will be limited to the epilepsy studies, discussion of adverse reactions will, where appropriate, include the pain and anxiety studies, because these provide additional information from controlled studies.
Chemical Structure, Formulations, And Methods For Determination In Body Fluids
Pregabalin (Lyrica) is S-3-(aminomethyl)-5-methylhexanoic acid, a compound with a molecular weight of 159.23.3 It is freely soluble in water. Although a structural analog of γ-aminobutyric acid (GABA) (Fig. 1), the additional side chain alters the distance between the GABA subunit terminals, with the net result that it is not a GABA mimetic. Gabapentin has a ring structure whereas pregabalin is a branched structure. Pregabalin development was the result of screening gabapentin analogs to develop an agent with greater antiepileptic efficacy, first by studies of inhibition of [3 H] gabapentin binding and then by animal studies.8 The S-isomer binds to the α-2-δ receptor, the same receptor involved in gabapentin-specific binding.12 Although freely soluble in water, at present approved formulations are only capsules. Development of liquid formulations are in progress, and the potential for a parenteral formulation exists. Therapeutic levels have not yet been established, but pregabalin can be assayed in plasma and other fluids using high-performance liquid chromatography.10
Pharmacology
Mechanism of Action
Like gabapentin, pregabalin binds to the α-2-δ subunit of voltage-gated calcium (P/Q) channels, and allosterically modulates calcium (Ca) influx, reducing it by up to 40%—with the greatest reduction occurring in states in which high neuronal activity is present.63 There are four subtypes of the α-2-δ subunit; pregabalin binds to subtypes 1 and 2 only. This modulation has been shown to reduce the presynaptic release of neurotransmitters, including noradrenaline,20,21 substance P,26 and the excitatory amino acid glutamate.19,42 These effects require binding of pregabalin at the α-2-δ subunit. Although the α-2-δ type 1 knockout is a lethal mutant, studies have been performed using the R217 A knock-in mice with α-2-δ type 1 receptors that have preserved function but markedly reduced binding of pregabalin,12,63 especially in the neocortex, hippocampus, basolateral amygdala, and spinal cord. In these mice, pregabalin has no benefit on pain, and has reduced anxiolytic and antiepileptic effects.28,63 These studies supplement the binding studies by showing that binding of pregabalin to the α-2-δ receptor is necessary for maximal therapeutic effects. In addition, in cultured hippocampal neurons, some evidence suggests that the effects of pregabalin on synaptic vesicular release are greatest before and early in the course of a train of electrical stimuli.42 In neuromuscular preparations, pregabalin reduced nerve-evoked muscle contractions by 16%.38 When pregabalin cannot bind to the α-2-δ receptors, as in mutant R217 A mice, it has no effect. There has been no demonstrated effect of pregabalin on L-type Ca channels in muscle. α-2-δ Type 1 and 2 receptors are present outside the central nervous system (CNS) (e. g., in spleen, liver, lung) but at much lower concentrations.61
Despite the structural similarity of pregabalin to the inhibitory amino acid GABA, the addition of the side chain changes the configuration of the GABA subunit, with the net result that no binding to pre- or postsynaptic GABA (A or B) receptors occurs.51,62 Pregabalin does not have any GABA mimetic properties, and there is no effect on GABA or chloride flux. In addition, brain GABA levels are not significantly altered by pregabalin,24 and there is no effect on GABA uptake or degradation. Studies to date have not revealed significant effects of pregabalin on other known antiepileptic mechanisms (e.g., voltage-dependent sodium conductances), nor is there evidence that pregabalin affects the postsynaptic responses of any neurotransmitters. Therefore, at present, pregabalin appears to have a specific binding site that modulates and reduces the presynaptic release of neurotransmitter through a mechanism of action that does not depend on any GABAergic actions.
Activity in Experimental Models of Epilepsy
The efficacy of pregabalin has been investigated in various animal models of epilepsy.63,64 Pregabalin potently inhibits tonic extensor seizures in the electroshock model (ED50 = 1.8 mg/kg, administered orally), and it also prevents tonic extensor seizures in the DBA/2 audiogenic mouse model (ED50 = 2.7 mg/kg, administered orally). The time course of action of pregabalin reflects the time course of radiolabeled drug in the CNS. In a kindled rat model of partial seizures, pregabalin prevented the more fully evolved stage 4 and 5 behavioral
seizures (lowest effective dose 10 mg/kg, intraperitoneally) and also reduced the duration of the electrographic discharges. All these are models of partial epilepsy or models that predict AED efficacy in partial epilepsy (maximum electroshock; MES).
seizures (lowest effective dose 10 mg/kg, intraperitoneally) and also reduced the duration of the electrographic discharges. All these are models of partial epilepsy or models that predict AED efficacy in partial epilepsy (maximum electroshock; MES).
At much higher doses (ED50 = 31 mg/kg, orally), pregabalin prevented seizures in the pentylenetetrazole (PTZ) model of epilepsy in mice. However, pregabalin did not prevent spontaneous absence-like seizures in the genetic absence epilepsy rats from Strasbourg (GAERS) and, in fact, seizures actually increased.64 At one time, it was thought that AED efficacy in the PTZ model predicted anti-absence efficacy, but more recently35,36 this has not been thought to be as reliable as the various genetic models for testing anti-absence efficacy of AEDs. Pregabalin does not appear to have significant anti-absence efficacy.
In one study4 using the lithium pilocarpine model of status epilepticus in rats, early treatment with pregabalin delayed the appearance of later epilepsy and reduced neuronal damage in the piriform cortex and entorhinal cortex but not the hippocampus. At present, however, insufficient evidence is available to conclude that pregabalin is neuroprotective or antiepileptogenic; additional studies are needed.
Clinical Pharmakokinetics
Absorption
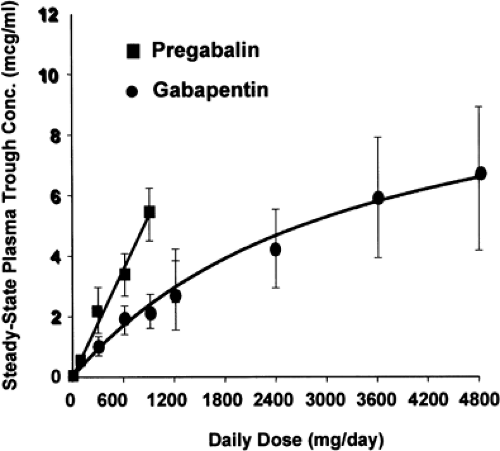
When fasting, oral bioavailability of pregabalin is excellent with >90% absorbed within 1 to 1.5 hours.14 Absorption is linear for both single doses up to 300 mg and multiple doses up to 900 mg/day (Fig. 2). This is an important difference and advantage of pregabalin compared with gabapentin, which is absorbed slowly, reaching peak doses in 3 to 4 hours. Although both compounds are transported across the gut and blood–brain barrier by an L-amino acid transport system, pregabalin has linear kinetics across and even above the clinical dosing range. This is quite different from the case with gabapentin, where even a 900 mg daily dose, given as 300 mg three times a day, has only 60% bioavailability, and a 3,600 mg daily dose has only 33% bioavailability when given as 1,200 mg three times a day.14 In comparing these two compounds, dose ranges of gabapentin from 900 mg to 3,600 mg per day and of 150 mg to 600 mg per day of pregabalin are commonly used, recognizing that these doses exceed the maximum approved dose for gabapentin in the United States (1,800 mg/day). Although the rate of pregabalin absorption is decreased slightly with food, the total amount absorbed is not affected.
Plasma Protein Binding and Distribution
Like gabapentin, pregabalin has negligible protein binding. The volume of distribution is approximately 0.5 L/kg.3
Metabolism
Elimination
Elimination of pregabalin is primarily renal; the drug has a mean elimination half-life of 6.3 hours. Pregabalin clearance is proportional to creatinine clearance (CLcr), and patients with renal insufficiency (CLcr <60 mL/min) will need appropriate dose reductions. A 50% reduction in dose is recommended
for patients with a CLCr between 30 and 60 mL/min.54 Because of pregabalin’s low protein binding, it will be removed by hemodialysis, and postdialysis adjustments may need to be made. Elderly patients with reduced CLcr will have proportionately reduced pregabalin clearance.
for patients with a CLCr between 30 and 60 mL/min.54 Because of pregabalin’s low protein binding, it will be removed by hemodialysis, and postdialysis adjustments may need to be made. Elderly patients with reduced CLcr will have proportionately reduced pregabalin clearance.
Relationships Between Plasma Concentration and Effects (Including the Value of Therapeutic Drug Monitoring)
Therapeutic levels for pregabalin have not been established, and there are no recommendations for monitoring of plasma levels. Because of the 6-hour half-life, steady-state levels are achieved rapidly after initiation or dose adjustment, in about 30 hours (five half-lives). Arroyo et al.6 found that steady-state doses of 150 mg/day produced mean plasma pregabalin levels of 1.27 μg/mL (range 0.29–2.84 μg/mL), and doses of 600 mg/day produced mean plasma pregabalin levels of 4.88 μg/mL (range 0.87–14.2 μg/mL).34 The dose range study of French et al. had similar findings.32 Beydoun et al.11 reported that 600 mg, given three times a day, produced mean plasma pregabalin levels of 5.82 μg/mL (range 0.38–18.2 μg/mL); twice-daily dosing produced mean plasma pregabalin levels of 6.84 μg/mL (range 0.32–14.80 μg/mL). Although twice-daily dosing resulted in higher peak levels and lower daytime trough levels than the three-times-daily regimen, morning trough levels were similar in both groups. Efficacy in reducing seizures correlated with these mean levels. Because of pregabalin’s rapid elimination, it would be expected that broad fluctuations in serum levels would occur even at steady state, and this is reflected in the broad ranges of plasma levels obtained for a given dose. This characteristic reduces the utility of random pregabalin sampling except to document compliance. It is possible that morning trough levels of pregabalin might be useful, but the range of such levels has not been established. It is not known how the pharmacokinetic profile of pregabalin in the serum relates to the pharmacodynamic profile in the CNS. The fact that efficacy with twice-daily dosing is not significantly different from three-times-daily dosing suggests the possibility of a pharmacodynamic effect distinct from plasma pharmacokinetics, but this has not been studied.
Efficacy
Pregabalin’s efficacy in reducing seizures has been studied in three double-blind controlled pivotal trials involving a total of 1,052 patients with intractable partial epilepsy.6,11,32 Although the criteria for entry into these trials were similar to other new AED trials—three seizures per month and failure with one AED—patients enrolled in the pivotal trials had frequent seizures and highly refractory epilepsy, with an average duration of epilepsy of 24 to 25 years, a mean monthly seizure frequency of 22, and a median monthly seizure frequency of 10. Approximately half the patients randomized to pregabalin were already taking two AEDs, and another quarter were on three AEDs, meaning that, during the actual trial, 75% of patients receiving active compound were taking three or four AEDs, including pregabalin. As predicted by pregabalin’s lack of known interactions with any AEDs, no significant effects on concomitant AEDs were observed.
One study32 was a dose-ranging study using from 50 mg/day to 600 mg/day given twice daily. A second study6 compared placebo with 150 mg/day and 600 mg/day of pregabalin given three times a day, and a third study11 directly compared 200 mg three times a day with 300 mg twice daily. The primary outcome variable in the first two studies was reduction of seizure frequency measured by the RRatio. The RRatio is defined as [(T – B) / (T + B)] × 100, where B is the patient’s 28-day baseline seizure frequency, and T is the patient’s 28-day seizure frequency during treatment. Although statistically rigorous, the RRatio yields numbers ranging from –100 to +100, with a value of –100 equivalent to no seizures and –33 equivalent to a 50% reduction in seizures. The RRatio was statistically significant for pregabalin doses of 150 mg/day and above. Doses of 50 mg/day did not produce significant reductions in seizures. Because these numbers are difficult to equate with clinical situations, most of the discussion here will focus on the more clinically relevant measures: seizure frequency and responder rates (here defined as a 50% reduction in seizures).
Converting the RRatio data to seizure frequency and responder rate yields similar results, although in one instance, the 150 mg/day dose in the Arroyo et al. study, the p value was not quite significant (p < 0.08). However, the number of seizures at this dose was significantly reduced. Indeed, the seizure numbers paralleled the illustrated responder rates. At the highest total daily dose, 600 mg per day with twice-daily or three-times-daily dosing, results were remarkably consistent from trial to trial, with responder rates of 43% and 51%, respectively. Although caution is warranted about extrapolating from one series of clinical trials to another, these responder rates at 600 mg/day of pregabalin compare favorably with the best results obtained with other new AEDs in similar patient populations characterized by highly refractory partial seizures.30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree