Primidone
Blaise F. D. Bourgeois
Introduction
Primidone has been in clinical use since 1952. Based on its chemical structure, primidone is not strictly a barbiturate. Therapeutically, however, it is appropriate to consider primi-done a barbiturate, because its clinical effect can be attributed in part to the phenobarbital produced by hepatic biotransformation. It has never been clearly established whether primidone is a phenobarbital prodrug, or whether treatment with primidone can be clinically distinguished from treatment with phenobarbital. Primidone does have independent antiepileptic activity in experimental models. If some of the therapeutic effect in humans can also be attributed to primidone, long-term therapy with primidone can be regarded as an obligatory combination therapy. In addition, independent antiepileptic activity has been demonstrated experimentally for the other main metabolite of primidone, phenylethylmalonamide (PEMA). These unique qualitative aspects of primidone biotransformation must be integrated into any discussion of its pharmacokinetics, pharmacodynamics, and clinical use.
Chemical Structure
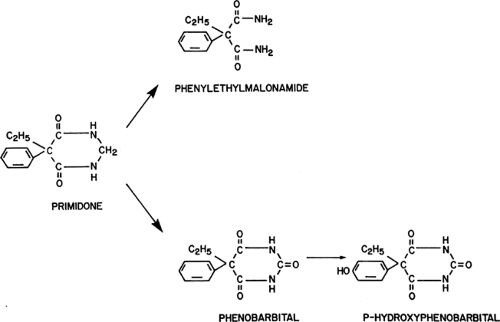
Primidone was first synthesized in 1952.8 Chemically, primi-done differs from phenobarbital only by the lack of the carbonyl group in position 2 of the pyrimidine ring, and it is therefore a desoxyphenobarbital (Fig. 1). The exact chemical composition is 5-ethyldihydro-5phenyl-4,6(1H,5H)-pyrimidinedione. It is very poorly soluble in water, somewhat soluble in ethanol, and virtually insoluble in organic solvents. Primidone can be synthesized by reduction of phenobarbital, desulfuration of thiophenobarbital, or ring closure of phenylethylmalonamide.1,10 The molecular weight of primidone is 218.25, and the factor to convert from milligrams to micromoles is 4.59. Thus, a concentration of 1 mg/L is equivalent to 4.59 μmol/L (Table 1). Because the pyrimidine ring of primidone contains only two carbonyl groups, and not the three carbonyl groups that characterize barbituric acid, primidone is not a barbiturate in the strict sense. However, it is appropriate to list the clinical use of primidone among the barbiturate therapies because of the biotransformation to phenobarbital, which accumulates to therapeutic levels during long-term administration of primidone to patients.
Pharmacology
The basic pharmacologic mechanism of action of primidone itself has received relatively little attention. One reason is that it remained uncertain for some time whether primidone itself actually has independent antiepileptic activity. In addition, primi-done is never present alone during long-term therapy, and at least one active metabolite, phenobarbital, is present after repeated administration in humans as well as in experimental animals. As discussed later, a second active metabolite, PEMA, may be involved in the overall pharmacodynamic effect of primidone. All the evidence regarding the individual pharmacodynamic properties of primidone, phenobarbital, and PEMA is derived from experiments in animals in which seizures were provoked. Because the metabolites begin to accumulate a few hours after administration of the first dose, a possible long-term protection by primidone alone against seizures occurring spontaneously cannot be assessed in humans. Independent anticonvulsant activity of primidone was first demonstrated in dogs; the animals were found to be protected against induced seizures at a lower concentration of phenobarbital when primi-done was also present.22 Subsequently, it was shown that rats were protected against induced seizures after a single dose of primidone before the active metabolites were detectable4; similar protection was achieved in mice when the biotransformation of primidone was delayed by the preadministration of a metabolic blocker.14,32 In terms of potency against seizures induced by maximal electroshock, primidone and phenobarbital are quite similar. Against seizures induced chemically by pentylenetetrazol or bicuculline, phenobarbital is effective but primidone is totally ineffective.14 This suggests that the experimental anticonvulsant spectra of primidone and phenobarbital differ and that the two compounds may be two different antiepileptic drugs (AEDs) with different mechanisms of action. The experimental anticonvulsant spectrum of primidone is similar to that of carbamazepine and phenytoin. Primidone and phenobarbital differ pharmacodynamically not only on the basis of their anticonvulsant spectrum, but also on the basis of their protective or therapeutic index. In terms of brain concentrations in mice, primidone was found to be 2.5 times less neurotoxic than phenobarbital, with a correspondingly higher therapeutic index.14 When phenobarbital and primidone were administered together in single-dose experiments in mice,15 their anticonvulsant activity was found to be supra-additive (potentiated), and their neurotoxic effect was found to be infra-additive. The best therapeutic index was achieved using a combination of the two drugs at a brain concentration ratio of 1:1. The results of interactions between primidone and phenobarbital obtained in mice were confirmed by experiments in amygdala-kindled rats. After single doses, the anticonvulsant effect of phenobarbital was potentiated by primidone, whereas side effects of phenobarbital, such as ataxia and muscle relaxation, were not increased by combined treatment with primidone.35
Table 1 Conversion | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
After direct administration, PEMA was found to have a relatively weak independent anticonvulsant activity in rats5 as well as in mice.14,32 When neurologic toxicity and protection against maximal electroshock-induced seizures were quantified in mice in terms of brain concentration,14 PEMA was found to be 16 times less potent than phenobarbital in terms of seizure protection, and eight times less potent than phenobarbital in terms of neurotoxicity. However, PEMA potentiated both the neurotoxic effect15 and the anticonvulsant effect5,14 of phenobarbital. However, a quantitative analysis of these experimental results, together with the blood levels measured in patients on long-term therapy with primidone, suggests that PEMA
probably contributes little or not at all to the antiepileptic effect or clinical toxicity in patients.
probably contributes little or not at all to the antiepileptic effect or clinical toxicity in patients.
Primidone’s basic anticonvulsant pharmacologic mechanism of action has been studied in mouse neurons in cell culture.36 Primidone was compared with phenobarbital for its effect on amino acid responses and on sustained, high-frequency firing. In contrast to phenobarbital, primidone had no effect on postsynaptic γ-aminobutyric acid (GABA) and glutamate responses at concentrations up to 50 μg/mL. However, both primidone and phenobarbital limited sustained, high-frequency, repetitive firing at relatively high concentrations (>50 μg/mL). Primidone and phenobarbital together limited sustained, high-frequency, repetitive firing at clinically relevant concentrations of 12 μg/mL for primidone and 20 μg/mL for phenobarbital. The authors concluded that primidone and phenobarbital may act synergistically to reduce sustained, high-frequency, repetitive firing.36 These in vitro findings are all in good agreement with the observations made in whole animals.
Clinical Pharmacokinetics
Absorption
Only oral preparations of primidone are available, and these include tablets and syrup. The extremely low solubility of pri-midone precludes its parenteral administration. Pharmacokinetic parameters of phenobarbital, the main active metabolite of primidone, are not reviewed here. The time to peak serum concentration of primidone after oral ingestion of tablets was found to be 2.7 hours24 and 3.2 hours23 in adult patients with epilepsy. In children, the peak blood concentration after a single oral dose was reached after 4 to 6 hours, and 72% to 123% of the total dose (average, 92%) was recovered in the urine as unchanged primidone and as metabolites.31 This suggests that the oral bioavailability of primidone is fairly complete. The concomitant administration of acetazolamide reduced the oral bioavailability of primidone.54 One report has suggested that the bioavailability of a generic preparation is lower than that of the brand product.57
Distribution and Protein Binding
Reported values for the volume of distribution of primidone vary: 0.54 L/kg (after acute administration)38 and 0.86 L/kg.43 In a study involving direct oral administration of PEMA, a volume of distribution of 0.69 L/kg was found for this metabolite of primidone.44 The protein binding of both primidone and PEMA in human plasma was found to be less than 10%.5,23,44
Primidone brain concentrations were lower than the simultaneous plasma concentrations, both in mice14,32 and in rats.4 In humans, variable brain-to-blood concentration ratios of pri-midone have been reported. An average brain-to-plasma concentration ratio of 87% was measured in a group of patients undergoing surgery for intractable epilepsy.28 In a different group of six patients, also undergoing epilepsy surgery, brain concentrations of primidone ranged between 0 and 2.2 μg/g, whereas the average plasma concentration was 6.3 μg/mL.32 Reported values for the cerebrospinal fluid-to-plasma ratio of primidone in humans range from 0.8 to 1.13.23,28,39,51 These values are similar to saliva-to-plasma concentration ratios for primidone,39,51 and they reflect the low affinity of primidone for plasma proteins. The brain penetration of PEMA seems to be good. In mice, brain concentrations of PEMA were found to be 93%32 and 77%14 of the simultaneous plasma levels.
Primidone brain concentrations were lower than the simultaneous plasma concentrations, both in mice14,32 and in rats.4 In humans, variable brain-to-blood concentration ratios of pri-midone have been reported. An average brain-to-plasma concentration ratio of 87% was measured in a group of patients undergoing surgery for intractable epilepsy.28 In a different group of six patients, also undergoing epilepsy surgery, brain concentrations of primidone ranged between 0 and 2.2 μg/g, whereas the average plasma concentration was 6.3 μg/mL.32 Reported values for the cerebrospinal fluid-to-plasma ratio of primidone in humans range from 0.8 to 1.13.23,28,39,51 These values are similar to saliva-to-plasma concentration ratios for primidone,39,51 and they reflect the low affinity of primidone for plasma proteins. The brain penetration of PEMA seems to be good. In mice, brain concentrations of PEMA were found to be 93%32 and 77%14 of the simultaneous plasma levels.
Metabolism
More than for any currently used AED, an understanding of the qualitative and quantitative aspects of primidone metabolism is a prerequisite for the rational clinical use of this drug. This is because, after repeated administration, clinically significant accumulation of at least one active metabolite, phenobarbital, and possibly of a second, PEMA, occurs (Fig. 1). Phenobarbital is further metabolized to p-hydroxyphenobarbital, an inactive metabolite. It would be desirable for optimal clinical use of primidone to know the relative antiepileptic potency and toxicity, as well as the expected ratios of blood levels, of primidone and its two active metabolites. The ratios of blood levels have been well studied, but, as outlined earlier, information on the pharmacodynamic aspects is limited mostly to data in animals; corresponding data are virtually impossible to obtain in humans. Following the introduction of primidone, PEMA was the first metabolite to be identified. It was found initially in rats7 and then in every species studied thereafter. A few years later, phenobarbital and p-hydroxyphenobarbital were identified,17 and this identification was soon followed by the first descriptions of clinical intoxications that were considered to be caused by the derived phenobarbital.45 Although other metabolites of primidone have been identified, they have no practical significance because of their low concentrations and lack of pharmacologic activity. The quantitative aspects of the biotransformation of primidone to phenobarbital and PEMA have been the object of several clinical studies. The fraction of primidone that is metabolized to phenobarbital was estimated by comparing the ratios of serum phenobarbital levels to the maintenance dose of either phenobarbital or primidone given as long-term therapy in the same patients. One such analysis suggested that 24.5% of the ingested primidone is converted to phenobarbital,41 based on the observation that the dose of primidone (in milligrams per kilogram per day) required to maintain a certain phenobarbital level was approximately four times higher than the dose of phenobarbital necessary to maintain the same level. The results of another study indicated that the primidone requirements for a given phenobarbital level are about five times higher than the corresponding phenobarbital dose.6 The extent of the biotransformation of primidone and the ratios of the blood levels of primidone and its metabolites are so sensitive to interactions with other AEDs that they are best discussed under that heading.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree