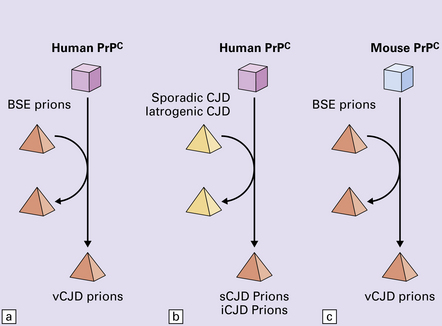
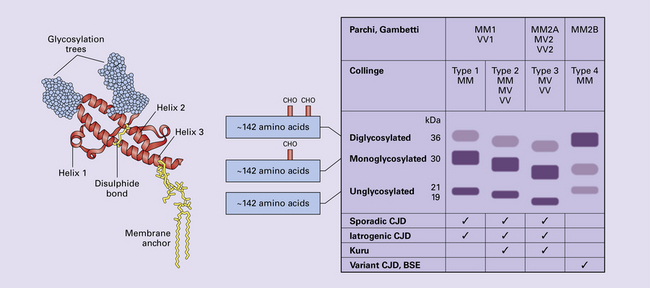
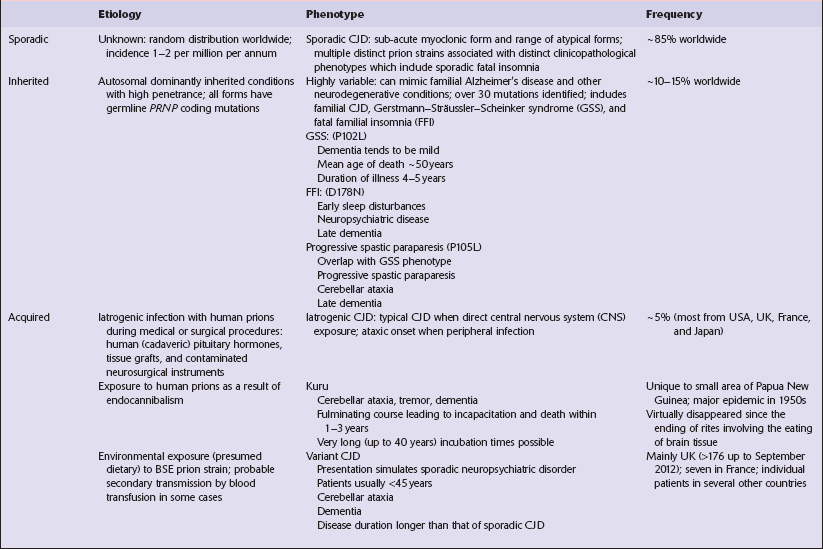
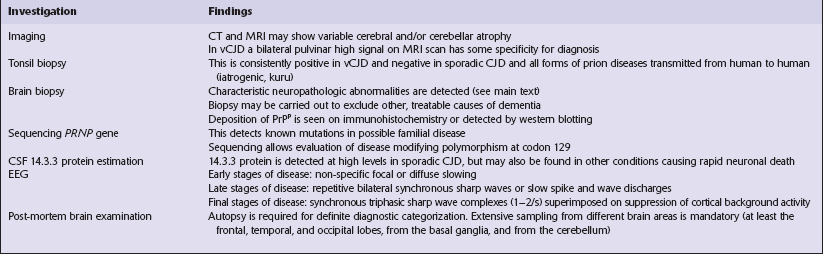
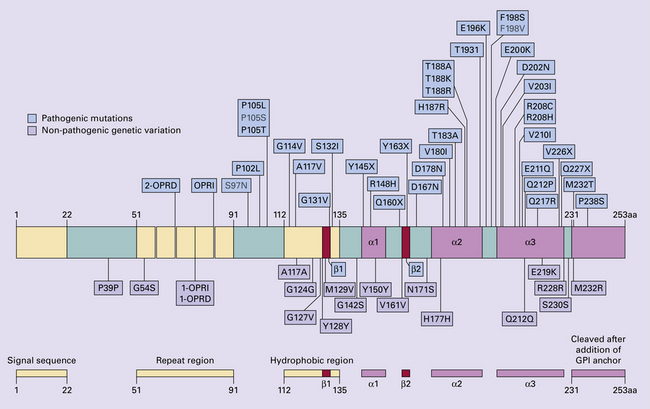
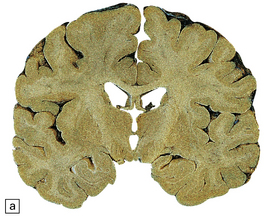
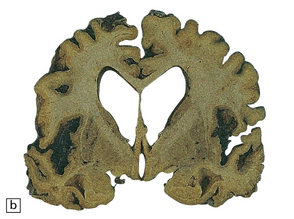
32 Common to all of these diseases are: The commonest phenotype of prion disease is CJD. Patients typically present with subtle motor signs, which herald severe cerebellar ataxia, and progress to global dementia in under 1 year. Criteria for the clinical diagnosis of CJD have been proposed and widely adopted (Table 32.1). Table 32.1 Clinical criteria for the diagnosis of CJD aDepression, anxiety, apathy, withdrawal, delusions. bThis includes both frank pain and/or unpleasant dysesthesia. cGeneralized triphasic periodic complexes at approximately one per second. dSpongiform change and extensive PrP deposition with florid plaques, throughout the cerebrum and cerebellum. Several phenotypes of prion disease other than CJD have been identified (Table 32.2). In all of these, the mainstay of diagnosis is clinical examination supplemented by additional radiologic, electrophysiologic and neuropathologic investigations (Table 32.3). The brain may appear macroscopically normal, even in cases with long clinical histories. Most cases, however, show some atrophy (Fig. 32.8) and this may be severe, with a reduction in brain weight to as low as 850 g. In such cases, ventricular enlargement is marked and the atrophy often includes the caudate nucleus and thalamus. The hippocampus may be relatively spared, even in cases with severe atrophy elsewhere. 32.8 CJD. (a) The brain may appear macroscopically normal, but there is usually some atrophy, as here. (b) Rarely, atrophy is marked. The histologic features of prion diseases are:
Prion diseases
 Creutzfeldt–Jakob disease (CJD) and variant Creutzfeldt–Jakob disease (vCJD).
Creutzfeldt–Jakob disease (CJD) and variant Creutzfeldt–Jakob disease (vCJD).
 Gerstmann–Sträussler–Scheinker disease (GSS).
Gerstmann–Sträussler–Scheinker disease (GSS).
 Fatal familial insomnia (FFI).
Fatal familial insomnia (FFI).
 The accumulation of an abnormal form of a cellular protein (prion protein).
The accumulation of an abnormal form of a cellular protein (prion protein).
 Neuronal death, synaptic loss, and microvacuolation (spongiform change) in the brain.
Neuronal death, synaptic loss, and microvacuolation (spongiform change) in the brain.

PATHOLOGY
MACROSCOPIC APPEARANCES
MICROSCOPIC APPEARANCES
 Spongiform change (intraneuronal vacuolation).
Spongiform change (intraneuronal vacuolation).
 Loss of synapses and subsequently of neurons.
Loss of synapses and subsequently of neurons.
 Paucity or absence of lymphocytic inflammation.
Paucity or absence of lymphocytic inflammation.
 Hyperphosphorylation of tau (immunohistochemical detection only).
Hyperphosphorylation of tau (immunohistochemical detection only).
 Accumulation of abnormal PrP (PrPSc) in various patterns:
Accumulation of abnormal PrP (PrPSc) in various patterns:
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Prion diseases
Only gold members can continue reading. Log In or Register to continue