Chapter 108 Prognostic Factors, Surgical Outcomes, and Guidelines for Managing Metastatic Spine Cancer
The spine is the most common site for skeletal metastasis.1 Approximately 5% to 10% of all cancer patients develop spine metastasis.2,3 Although Jaffee1 reported in 1958 a 70% incidence of vertebral metastasis, others asserted that spinal involvement ranges from 33.4% to 56%.4–6 Spinal cord compression occurs in 10% to 25% of treated patients with preexisting spinal metastasis.2,6–12 It is well established that if metastatic vertebral disease is left untreated, it may lead to paraplegia,13–16 which is the most dreadful complication of metastatic vertebral disease. In this situation, surgery plays an important role. Several surgical options exist; however, their indications and outcomes must be scrutinized for better medical care. The discovery of vertebral metastases intensifies an already complex situation requiring highly technical management, sustained coherence of the entire care-provider team, and their awareness of the patient’s increased need to be heard, informed, and supported.
Surgical Outcomes
The following are essential when evaluating surgical outcomes for vertebral metastases:
According to published reports, the anticipated improvement after surgery is expected to range from 55% to 87% of cases as a result of overall upgrade of neurologic deficit (Table 108-1); 36% to 89% because of restoration of sphincter control (Table 108-2); 47% to 100% due to pain relief (Table 108-3); and 40% to 100% as a result of restoration of ambulation (Table 108-4). Apparently restoration of ambulation after dorsal decompression alone fares worse. The anticipated survival rates are depicted in Table 108-5. The complication rates range from 12% to 32% (Table 108-6). In our series of 70 patients, the complication rate was 25%, including two deaths. In one case, surgery was aborted, while in progress, because of massive intraoperative bleeding, despite preoperative transarterial embolization of the region.17 Other complications were profuse bleeding in nine patients, infections in three, wound dehiscence in three, and incomplete surgery in three patients. The 4- to 6-week mortality rates after surgery, reoperation rates for complications, and local recurrences after surgery are listed in Tables 108-7, 108-8, and 108-9, respectively. Local tumor recurrence is more likely to occur in patients with certain tumors and particularly in those for whom chemotherapy and radiation therapy are ineffective. This is particularly the case in such cancers as renal cell carcinoma, in which the reported recurrence is expected to be as high as 40% to 50%.18–21 The overall patient satisfaction was rated in the range of 55% to 80%.18,22
TABLE 108-1 Reported Overall Neurologic Improvement after Surgery
| Study | Improvement |
|---|---|
| Kostuik J et al.68 | 55% |
| Ernstberger T et al.80 | 57% |
| Weigel B et al.18 | 58% |
| King GJ et al.19 | 60% |
| Villavicencio AT et al.81 | 60% |
| Rompe JD et al.82 | 63% |
| Atanasiu JP et al.30 | 64% |
| O’Neil J et al.32 | 68% |
| Harrington KD37 | 68% |
| Hatrick N et al.83 | 69% |
| Tomita T et al.72 | 73% |
| Hammerberg KW66 | 74% |
| Bauer HCF84 | 76% |
| Gokalsan ZL et al.46 | 76% |
| Onimus M et al.85 | 79% |
| Hosono N et al.86 | 81% |
| King et al.19 | 88% |
| Hertlein H et al.87 | 88% |
| Shimizu K et al.34 | 87% |
TABLE 108-2 Restoration of Sphincter Control after Surgery
| Study | Successful Restoration |
|---|---|
| King GJ et al.19 | 36% |
| Kocialkowski A, Webb JK88 | 43% |
| Kostuik J et al.68 | 47% |
| Sinardet D et al.89 | 51% |
| Tomita T et al.72 | 77% |
| Klekamp J, Samii H31 | 89% |
| Study | Success Rate |
|---|---|
| Sinardet D et al.89 | 47% |
| Touboul E et al.90 | 71% |
| Hirabayashi H et al.25 | 77% |
| Kocialkowski A, Webb JK88 | 79% |
| Kostuik J et al.68 | 81% |
| Sundaresan N et al.91 | 85% |
| Ernstberger T et al.80 | 85% |
| Villavicencio AT et al.81 | 85% |
| King GJ et al.19 | 88% |
| Weigel B et al.18 | 89% |
| Hatrick N et al.83 | 90% |
| Hammerberg KW66 | 91% |
| Gokalsan ZL et al.46 | 92% |
| Hertlein H et al.87 | 92% |
| Harrington KD37 | 94% |
| O’Neil J et al.32 | 94% |
| Hosono N et al.86 | 94% |
| Atanasiu JP et al.30 | 95% |
| Tomita T et al.72 | 95% |
| Shimizu K et al.34 | 100% |
| Bilsky MH et al.92 | 100% |
TABLE 108-4 Restoration of Ambulation after Ventral or Dorsal Approach
| Study | Successful Restoration |
|---|---|
| Livingston KE, Perrin RG93 | 40% |
| Kostuik J et al.68 | 44% |
| Gokalsan ZL et al.46 | 51% |
| Sinardet D et al.89 | 52% |
| Tomita T et al.72 | 60% |
| Patchel RA et al.94 | 62% |
| Hosono N et al.86 | 64% |
| King GJ et al.19 | 64% |
| Weigel B et al.18 | 70% |
| Onimus M et al.85 | 70% |
| Hammerberg KW66 | 76% |
| Hatrick NC et al.83 | 78% |
| Siegal T, Siegal T95 | 80% |
| O’Neil J et al.32 | 82% |
| Villavicencio AT et al.81 | 100% |
| Sundaresan N et al.91,96 | 100% |
| Restoration of Ambulation after Laminectomy Alone | |
| Klekamp J, Samii H31 | 23% |
| Kennady JC, Stern WE97 | 23% |
| Schoeggl A et al.98 | 33% |
| Wright RL99 | 35% |
| White WA et al.100 | 36% |
| Siegal T, Siegal T95 | 47% |
TABLE 108-5 Anticipated Survival Rates after Surgery
| Study | Survival Rate |
|---|---|
| 3 month | |
| Kocialkowski A, Webb JK88 | 50.0% |
| Wai EK et al.52 | 76.0% |
| 6 month | |
| Klekamp J, Samii H31 | 58.8% |
| Rompe JD et al.82 | 72.0% |
| Hosono N et al.86 | 78.0% |
| 9 month | |
| Falicov A et al.33 | 50.0% |
| 12 month | |
| Kocialkowski A, Webb JK88 | 25.0% |
| Klekamp J, Samii H31 | 48.8% |
| Rompe JD et al.82 | 50.0% |
| Villavicencio AT et al.81 | 65.0% |
| Gokalsan ZL et al.46 | 62.0% |
| 19 month | |
| Rompe JD et al.82 | 4.7% |
| 60 month | |
| Klekamp J, Samii H31 | 5% |
| Median month | |
| Schoeggl A et al.98 | 6.5% |
| Sioutos PJ et al.35 | 10% |
| Hirabayashi H et al.25 | 10.6% |
| Lewandrowski KU et al.101 | 14% |
| Sundaresan N et al.102 | 30% |
| Mean month | |
| Chataigner H, Onimus M20 | 8% |
| Hertlein H et al.87 | 8.5% |
| O’Neil J et al.32 | 9% |
| Livingston KE, Perrin RG93 | 9% |
| Atanasiu JP et al.30 | 11% |
| Ernstberger T et al.80 | 15.6% |
| Weigel B et al.18 | 13.1% |
| Wise JJ et al.67 | 15.9% |
| Cahill DW, Kumar R103 | 15.9% |
| Kocialkowski A, Webb JK88 | 12.0% |
TABLE 108-6 Complication Rates
| Study | Percentage Experiencing Complications |
|---|---|
| Bilsky MH et al.7 | 12% |
| Wai EK et al.52 | 12% |
| Hosono N et al.86 | 16% |
| Weigel B et al.18 | 19% |
| Jansson KA, Bauer HC104 | 20% |
| Wise JJ et al.67 | 25% |
| Klimo P Jr et al. (review of 24 surgical reports)105 | 23% |
| Falicov A et al.33 | 29% |
| Gokalsan ZL et al.46 | 32% |
TABLE 108-7 Postsurgery Mortality Rates (4 to 6 weeks)
| Study | Mortality Rate |
|---|---|
| Gokalsan ZL et al.46 | 3.0% |
| Klimo PJr et al.105 | 6.3% |
| Wise JJ et al.67 | 6.0% |
| Siegal TJ et al.106 | 6.0% |
| Weigel B et al.18 | 7.0% |
| Sundaresan N et al.96 | 8.0% |
| Harrington KD37 | 8.0% |
| Sinardet D et al.89 | 9.0% |
| Sioutos PJ et al.35 | 11.0% |
| Turner PL et al.78 | 10.0% |
| Hertlein H et al.87 | 10.0% |
| Chataigner H, Onimus M20 | 10.2% |
| Bilsky MH et al.92 | 12.0% |
| Jansson KA, Bauer HC104 | 13.0% |
| Kocialkowski A, Webb JK88 | 16.0% |
| Fidler MW107 | 18.0% |
TABLE 108-8 Reoperation Rates for Complications or Local Recurrences
| Study | Percentage Undergoing Reoperation |
|---|---|
| Weigel B et al.18 | 7.0% |
| Jansson KA, Bauer HC104 | 10.2% |
| Hertlein H et al.87 | 12.1% |
| Bauer HCF84 | 24.0% |
| Chataigner H, Onimus M20 | 15.8% |
| King GJ et al.19 | 56.2% |
TABLE 108-9 Local Recurrences after Surgery
| Study | Percentage Experiencing Recurrence |
|---|---|
| Falicov A et al.33 | 3.5% |
| Review of 9 surgical reports | 8.0% |
| Klimo P Jr et al.105 | |
| Bilsky MH et al.92 | |
| Foumey DR et al.108 | |
| Hammerberg KW66 | |
| Hosono N et al.86 | |
| King GJ et al.19 | |
| Muhlbauer M et al.109 | |
| Siegal T et al.106 | |
| Weigel B et al.18 | |
| Chataigner H, Onimus M20 | 8.4% |
| Nazarian S110 | 11.0% |
| King GJ et al.19 | 12.1% |
| Hirabayashi H et al.25 | 21.0% |
| Weigel B et al.18 | 22.0% |
| Bridwell KH et al.71 (after laminectomy) | 31.6% |
| Sundaresan N et al.102 | 32.0% |
| Hertlein H et al.87 | 49.0% |
Prognostic Factors
It has been observed that certain factors, irrespective of treatment selection, may influence the prognosis when malignant tumors develop spinal metastases. The original claim that the histopathology of the tumor had no bearing on the ultimate prognosis23 has not been duplicated by others.
Origin of Tumor
The origin of the primary tumors was postulated by several authors to influence the prognosis. Thus, metastatic vertebral tumors from unsuspected adenocarcinoma, stomach, esophagus, pancreas, and lungs, portend the worst possible prognosis, whereas carcinoid, thyroid, breast, prostate, and myeloma carry the best prognosis. Distinction should also be made between the different types of lung cancer.24 In general, non–small cell lung cancer (NSCLC) patients with spinal metastases had survival rates of 37.1%, 14.6%, and 2.1% at follow-up of 6 months, 1 year, and 2 years, respectively. The median survival time was 4.5 months, and the mean, 6.2 months. However, for small-cell lung cancer (SCLC), the corresponding survival rates were 36.8%, 5.3%, and 0% at 6 months, 1 year, and 2 years follow-up, respectively. In both NSCLC and SCLC with spinal metastases, the presence of hypercalcemia or hypoalbuminemia was indicative of a gloomy prognosis, with a survival period of less than 3 months.24
The aggressiveness of cancer can also be classified as slow growth (breast, thyroid, prostate), moderate growth (kidney, uterus), and rapid growth (lung, stomach, liver, colon, unknown).25–29
Concurrent Visceral Metastases
The influence on survival with visceral metastasis was also observed by Tokuhashi et al.28 and Tomita et al.29 The average survival with no visceral metastases was 36.8 months (range, 5–84 months), treatable visceral metastases, 16.5 months (4–31 months), and untreatable visceral metastases, 8.9 months (range, 1–24 months). The grave prognostic factor of extraskeletal metastatic lesions on the survival rate is also in agreement with Weigel et al.,18 who demonstrated that a patient survival period without extraskeletal metastases at surgery was significantly longer than with extraskeletal metastases (23.5 months vs. 5.8 months; P < .0001).
Location of Spinal Lesion
The location of the metastatic lesion was also implicated as a survival factor. Atanasiu et al.30 reported that lesions in the upper cervical spine had an adverse effect on life expectancy, with average survival of 1.8 months; however, this was challenged by Klekamp and Samii,31 who found no differences in prognosis between the upper and lower spine.
Number of Affected Vertebrae
The number of affected vertebrae also had an influence on survival. Patients with single-level involvement had a better survival rate (average survival, 12.9 months) than did patients with multiple-level involvement (average, 7.9 months),28,32,33 even after effective surgical treatment.34
Neurologic Deficit
Neurologic dysfunction was observed to be associated with survival rates. Some believe that ambulatory patients survive longer than nonambulatory patients with sphincter incontinence.19,35 The state of sphincter incontinence as a bad prognostic sign is also emphasized by some.36 The rate of neurologic deterioration was related to prognosis; slow progression had a good prognosis as opposed to rapid progression, which was correlated with dismal results. The latter was compounded when the onset of treatment was delayed.36,37 However, the notion of preoperative intact neurologic status (ambulatory vs. nonambulatory) as a predictor of longer survival was challenged by others.18,38
Likewise, timing for adjunctive radiation treatment (preoperative radiation vs. postoperative radiation) was also questioned as a significant prognostic predictor for outcomes.39 Weigel18 and Hirabayashi et al.25 emphasized that postoperative ambulation definitely has a positive prognostic influence on survival, rather than preoperative ambulation.
Timing of Surgery
The timing of treatment can also influence the results. Some authors36,37 observed that delayed onset of treatment may compromise the effectiveness of neurologic recovery. More specifically, Fürstenberg et al.40 addressed the timing of surgery and concluded that the effectiveness of decompression, when undertaken less than 48 hours after the development of symptoms, was significantly better for neurologic recovery (71.4%, unchanged 28.6%), compared with those with delayed surgical treatment (improvement 28.6%, unchanged 42.8%, deterioration 28.6%). Furthermore, they noted that normal bladder function may be considered a good prognostic factor for neurologic recovery after appropriate decompression.
Age
Weigel et al.18 also reported that the patient age can be considered as a prognostic factor for survival, because patients younger than 60 years survived significantly longer than older patients (20.1 months vs. 6.2 months; P = .028). Age as a prognostic factor has not been incorporated into the Tokuhashi scoring system and needs to be duplicated by others.
More Than Two Prognostic Factors
A report indicates that when more than two of the “prognostic factors” are present, they have a compounding adverse effect on survival.35 For example, lung cancer, neurologic deficit, and involvement of multiple vertebrae in the same patient would have a greater adverse effect on survival than the combined effect of the same type of lung cancer involving a single vertebra and without neurologic deficit. However, these findings have not been substantiated by others. When estimating survival, one should take into consideration that, invariably, most of the data from different reports were based on metastatic tumor of the spine that became symptomatic.
Instruments for Outcomes Assessment
Evaluation of patient functional status should be based on (1) pain assessment, (2) profile of mood states, and (3) overall performance status. Because performance depends not only on pain and disability but also on neurologic status, a detailed neurologic assessment is an integral part of the evaluation of these patients and provides reliable and straightforward clinical documentation. However, the assessment of pain disability and bodily functional performance of the patient can be appreciated only through psychometric instruments, which should be simple and reproducible.41
Because treatment is seldom curative in metastatic disease,42 the treating physician should keep in mind that patients with vertebral metastasis differ from noncancer patients. Therefore, the sensitivity of these instruments is limited to monitoring changes of the patient’s feelings of well-being.43,44
Pain is an alarming consequence of vertebral metastatic disease. Specific efforts should be made to avoid crude assessment and try to quantify and locate the pain. The McGill Pain Questionnaire is a sensitive tool. A visual analogue scale (VAS) for pain measurement combined with a pain diagram that indicates the site of pain and its spread is a simple and reliable method for quantifying and depicting pain. The VAS, although a subjective assessment of pain, has been shown to be more reliable than other types of pain assessment.45
A distinction should be made between radicular pain and axial pain. Even the surgical approach may be responsible for severe pain. A report46 detailing outcomes of pain demonstrated that in 72 patients who underwent a ventral approach through thoracotomy, 90% had postoperative pain (only 23% achieved complete resolution, 60% had significant improvement, and 8%, no change or worsening of thoracotomy pain). Quantification of pain is useful in monitoring the effectiveness of pain management, as well as in helping establish pain-control goals for the individual patient.47
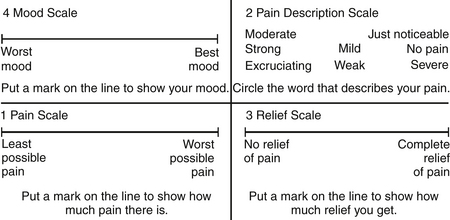
Cancer patients often have emotional and psychological problems. Because anxiety and depression have a direct correlation with pain intensity, these two factors may need to be addressed. The Zung Self-Rating Anxiety Scale48 and the Hamilton Depression Scale49 are suitable instruments in this situation. The Memorial Pain Assessment Card (MPAC)50 (Fig. 108-1) is a modified visual analogue with multidimensional characteristics, practically equivalent to a full assessment combining the McGill Pain Questionnaire, the Hamilton Depression Rating Scale (HDRS), and the Zung Self-Rating Anxiety Scale.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree