CPS, complex partial seizure; GTC, generalized tonic–clonic seizure; MMPI II, Minnesota Multiphasic Personality Inventory-2.
When shown a video of ES and PNES, neurologists are highly accurate (89%) in distinguishing the two types of seizures (45). Importantly, neurologists are accurate in diagnosing PNES by video when considering the constellation of signs. However, when attempting to make the diagnosis based on one sign on video, they are not as accurate (41). Likewise, when presented with the history alone, neurologists have a high sensitivity for ES, but specificity was only 50%, with simple partial epileptic seizures and PNES being frequently missed (15). With the nearly ubiquitous availability of video cameras on smart phones, patients and their significant others may be able to provide the data for a clinician to help make the diagnosis. The primary limitation would be the quality of the video and the strong likelihood that only part of the PNES would be captured without the beginning of the event.
Laboratory and Neuroimaging
Use of prolactin (PRL) level has been extensively reviewed in an American Academy of Neurology (AAN) technical guideline report (47). Pooled data, of studies distinguishing both complex partial and generalized seizures from PNES, demonstrate that PRL are highly specific (95.9% to 96.3%) but not that sensitive (46.1% to 60%). PRL fluctuates with circadian rhythm, time of day, quality of sleep, and with syncope. Most studies demonstrating utility of PRL level measured baseline levels and within 30 minutes after the seizure since levels begin to attenuate within hours of the seizure. AAN guidelines suggest that PRL levels should be drawn within 20 minutes of the seizure to be most useful. Criteria for determining elevated PRL have included a 300% increase from baseline; reaching >25 ng/mL; and reaching > 32 ng/mL for females and >23 ng/mL for males. A rise in PRL level does not occur in simple partial epileptic seizures. Syncope has been shown to elevate PRL levels; therefore, PRL are likely not useful to distinguish between ES and syncope.
The majority of structural neuroimaging studies in people with PNES are within normal limits, although abnormalities are present in a significant minority of studies (49). While there is an increased interest in studying neuroimaging changes in people with somatization and dissociative disorders (50–52), including PNES (53,54), neuroimaging does not distinguish between ES and PNES. Two studies have shown that changes in interictal and postictal single photon emission computed tomography (SPECT) may be useful when semiology of PNES is atypical or VEEG is unclear (48,55).
Neurophysiology/EEG Monitoring
Several studies have demonstrated that nonspecific interictal EEG abnormalities may be seen in people with PNES that may be due to overreading of normal variants on the EEG (56), comorbid medical and psychiatric issues (such as traumatic brain injury or psychotropic medications) (57), and misdiagnosis of frontal lobe seizures (58). Criteria for VEEG diagnosis have also been debated. Research studies have varied in how many of the typical events should be captured and how to interpret interictal epileptiform activity (31). Studies vary regarding how many episodes should be captured to determine the diagnosis, whether interictal discharges rule out PNES, whether to include preictal and postictal EEG changes as part of the criteria, and the how to use psychogenic semiology in the diagnosis (31). The gold standard is to capture a PNES that is typical of the events the patient experiences in the community. The entire event should be captured, and there should be ideally no epileptiform activity on VEEG before, during, or after the event. In addition, the semiology can provide helpful information (listed in Table 40.1). The heart rate, for example, tends to accelerate relative to baseline during ES but not PNES and can distinguish between the two with a sensitivity of 83% and specificity of 96% (59). Postictal agitation and confusion are seen in both ES and PNES (60); however, PNES is distinguished from ES postictally by shallow rapid breathing as opposed to deep, heavy breathing in ES (61). Recently, the International League Against Epilepsy PNES Task Force proposed criteria for diagnostic levels of certainty for PNES to offer guidance in events that cannot be captured by VEEG either because the technology is not available or because events are too infrequent to expect capture (Table 40.2) (30). The three criteria involve history, semiology, and EEG and levels include “Documented,” “Clinically established,” “Probable,” and “Possible PNES.”
Table 40.2 Overview of Proposed Diagnostic Levels of Certainty for PNES
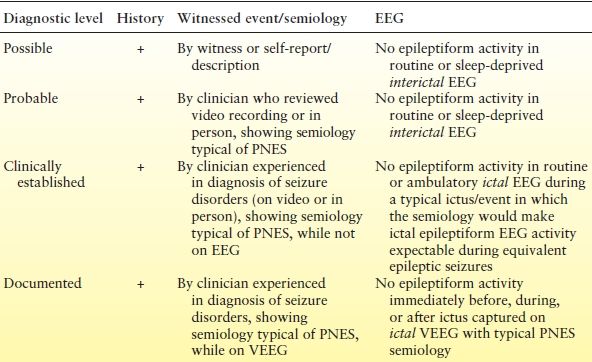
As noted in the text, additional tests may affect the certainty of the diagnosis—for instance, self-protective maneuvers or forced eye closure during unresponsiveness or normal postictal prolactin levels with convulsive seizures.
+, history characteristics consistent with PNES; EEG, electroencephalogram.
Reproduced with permission from LaFrance WC Jr, Baker GA, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia. 2013;54(11):2005–2018.
COURSE OF ILLNESS
Due to the delay in the diagnosis of PNES, most (if not all) people with PNES experience some stigma and restructure their lives around the diagnosis of epilepsy. Furthermore, the delay in diagnosis and treatment as epileptic seizures frequently results in unnecessary use of AEDs, incurring the risk of side effects, as well as the high cost of chronic use (16). Less commonly, patients may be inappropriately referred for neurosurgical interventions for “medically intractable epilepsy” (62). Standard medical care (SMC) at this time is limited to communicating the diagnosis of PNES, tapering AEDs in patients with PNES only and referring to a psychiatrist or psychologist for psychiatric comorbidities (63). Although a few small studies have shown marginal improvement in the frequency of PNES with only communicating the diagnosis, SMC has not shown significant improvement in depression and anxiety symptoms, quality of life measures, utilization of health care resources, dependence of state welfare programs, or employment status. Unlike people with epilepsy who have improvement in quality of life (QOL) with seizure remission (64), in the minority of patients (approximately 30%) who experience PNES remission with SMC, utilization of health care services, quality of life, anxiety, depression, somatization, and unemployment status are not better than those who continue to experience PNES (65). People who suffer from PNES die earlier than the general population (66) and have a high rate of self-injury, as well as suicide attempts (35). Therefore, overall people with PNES have worse outcomes than people with ES. As reviewed elsewhere (67), factors that are associated with lower remission of PNES include the following: adulthood during the age of PNES onset (68), female gender (69), receiving social security benefits (70), lower education (8,71), unemployment (8,70), difficulty forming relationships (37), not accepting the diagnosis of PNES (70), people with more violent psychogenic seizure semiology (72), and higher level of psychopathology (69,73). In particular, people with delusions, paranoia, and bizarre behavior are less likely to achieve PNES control (74). Personality characteristics, including lower emotional dysregulation, inhibitedness, and compulsivity scores, are associated with better outcomes (8).
TREATMENT
Prior editions of this chapter have almost exclusively focused on PNES as a differential diagnosis. However, the emerging literature (75–77) on treatment warrants review of the increase in evidence-based therapies. Once the diagnosis of PNES is made, there are three critical phases to treatment of PNES: presentation of the diagnosis, gaining control over the symptoms, and maintenance therapy (77). Some neurologists argue that providing the diagnosis of PNES itself is (at least part of) the treatment for PNES, but as discussed SMC is associated with poor outcome. Open-labeled psychological interventions, including psychodynamically oriented, as well as cognitive behavioral therapies (CBTs) (78,79), and pilot pharmacologic clinical trials (80) have demonstrated improved outcomes. Two pilot studies of CBT for PNES focused on PNES-related trigger prevention has resulted in seizure freedom of 25% (78) and 65% (81) and a >50% reduction of seizure frequency in 76% and 81% of subjects enrolled, respectively.
Pharmacologic trials for PNES include the use of psychotropic medications and analgesics (82). In a double-blinded, placebo-controlled, 12-week clinical trial of sertraline, 38 subjects were enrolled and 26 (68%) completed the study (80). Subjects who received sertraline had a 45% reduction in seizures from baseline, compared to an 8% increase in those who received placebo. No significant difference was seen in depression, anxiety, impulsivity, somatic symptoms, QOL, and psychosocial functioning scores. This feasibility study was not powered to demonstrate efficacy, but interestingly showed improvement in seizure frequency independent of mood and anxiety symptoms. A 5-month, open-label prospective study of venlafaxine required subjects to have a comorbid DSM-IV diagnosis of an anxiety and/or unipolar depressive disorder (83). Of the 19 subjects that completed the trial, 8 were seizure free at 5 months, 15 (88%) had >50% reduction in frequency of seizures, 11 (65%) had >50% reduction in depression scores, and 7 (41%) had >50% reduction in anxiety scores. Absence of a control arm makes it difficult to determine how much of the improvement was due to the medication. A recent pilot multicenter randomized trial comparing a workbook-based psychotherapy (84), workbook psychotherapy plus SSRI, SSRI alone, and SMC revealed significant seizure reduction and improvement in comorbidities in the psychotherapy and psychotherapy plus SSRI arms and no improvement in the SMC arm (85). The study illustrates that effective treatments exist for PNES and that the current standard of treatment (usually supportive psychotherapy) is not adequate in treating patients with PNES.
CONCLUSION
Much has been learned recently about the nature and consequences of PNES, which have been documented in the medical literature for centuries. As information is increasingly available on the characteristics and treatments for PNES, neurologists, mental health providers, and other health care professionals not only should consider PNES in the differential diagnosis of paroxysms but should also actively be engaged in providing care for these patients. The risk of unnecessary AED, emergency room visits, hospital admissions, unnecessary procedures, and self-injury is exceedingly high. Clinicians may play a central role in not only diagnosing PNES but also referring patients to appropriate mental health treatment. Neurologic clinics and epilepsy centers may even consider collaborating closely with mental health professionals to develop multidisciplinary care for conversion disorders beyond PNES, such as psychogenic movement disorders or other psychosomatic complaints (86). Hope for improvement in PNES is present not only for patients and family members but also for neurologists who evaluate and care for this challenging population.
References
1. Stone J, Campbell K, Sharma N, et al. What should we call pseudoseizures? The patient’s perspective. Seizure. 2003;12(8):568–572.
2. Benbadis SR. Psychogenic nonepileptic “seizures” or “attacks”? It’s not just semantics: attacks. Neurology. 2010;75(1):84–86.
3. LaFrance WC Jr. Psychogenic nonepileptic “seizures” or “attacks”? It’s not just semantics: seizures. Neurology. 2010;75(1):87–88.
4. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: conversion disorder. Am J Psychiatry. 2010;167(6):626–627.
5. Brown RJ, Cardena E, Nijenhuis E, et al. Should conversion disorder be reclassified as a dissociative disorder in DSM V? Psychosomatics. 2007;48(5): 369–378.
6. Laria AJ, Lewis-Fernandez, R. The professional fragmentation of experience in the study of dissociation, somatization, and culture. J Trauma Dissociation. 2001;2(3):17–47.
7. Ettinger A, Devinsky O, Weisbrot D, et al. Headaches and other pain symptoms among patients with psychogenic non-epileptic seizures. Seizure. 1999;8(7):424–426.
8. Reuber M, Pukrop R, Bauer J, et al. Outcome in psychogenic nonepileptic seizures: 1 to 10-year follow-up in 164 patients. Ann Neurol. 2003; 53(3):305–311.
9. Gazzola DM, Carlson C, Rugino A, et al. Psychogenic nonepileptic seizures and chronic pain: a retrospective case-controlled study. Epilepsy Behav. 2012;25:662–665.
10. Reuber M, House A, Pukrop R, et al. Somatization, dissociation and general psychopathology in patients with psychogenic non-epileptic seizures. Epilepsy Res. 2003;57(2–3):159–167.
11. Kuyk J, Spinhoven P, van Emde Boas W
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








