Radiosurgical Management of Cerebrovascular Malformations in Children Arteriovenous malformations (AVMs) of the brain are a common cause of hemorrhagic stroke in the pediatric population. In contrast to adults, in whom AVMs cause hemorrhage, seizures, headaches, and progressive neurologic deficits, the majority of children have an initial brain hemorrhage.1,2 Because of this risk for hemorrhage and its attendant morbidity and mortality, as well as the lifelong risks of an AVM should it go untreated, the majority of children with brain AVMs undergo some form of therapy. Rather than conservative management, treatment can include microsurgical resection, embolization, or stereotactic radiosurgery, alone or in combination. Although they are considered to be congenital, it is unclear when brain AVMs actually develop.3 They are thought to develop in the fetus, but the rarity of presentation during the neonatal period has prompted some investigators to question whether AVM development only begins in utero, then progresses during the first years of life.4 This possibility also has been raised by various authors, who have noted the recurrence of childhood AVMs despite total resection confirmed by angiography.4–6 Others have noted the association between vascular malformations and tumors.7 Some are associated with aneurysms, venous sinus anomalies, or fistulas.8–13 Certainly some AVMs are detected shortly after birth, such as large parenchymal or vein of Galen malformations that present with congestive heart failure.14–16 Most AVMs are detected between the ages of 20 and 40 years, which may suggest latency in the evolution of the malformation. Perhaps some do evolve in their angioarchitecture, eventually posing a greater and greater risk for the patient.6 Nevertheless, 20% of AVMs entered into the first cooperative AVM study were diagnosed before the age of 20 years, and thus the childhood presentation of an AVM is not uncommon.17 In general, AVMs cause a significant proportion of pediatric strokes and are three times more likely than saccular aneurysms to cause intracerebral hemorrhage in persons younger than 18 years.2 Matson declared that AVMs were the “most frequent abnormality of the intracranial circulation in childhood.”18 Because most children with AVMs undergo treatment, reports of untreated patients are too few in number for the natural history of untreated pediatric AVMs to be fully understood. In retrospective series, most authors found a hemorrhage rate at presentation higher than that reported for adults, usually in the range of 80%.19,20 In the 40-year Toronto experience published in 1992, Kondziolka et al reported on 132 children. Of these, 27 were managed conservatively, and death followed catastrophic hemorrhage in 13 of them. Of eight evaluable conservatively managed children, six were normal, one had a seizure disorder, and one had a persistent neurologic deficit.6 In the entire Toronto series, the mortality rate from AVM hemorrhage was 25%. This high rate may have been related to a higher incidence of posterior fossa AVMs, in which the initial hemorrhage was often lethal. In the general referral population of the Toronto series, 31 children (24%) had a posterior fossa AVM, a feature that varied significantly from the cooperative study (32 of 453; p < 0.0001). At present, there is no evidence that AVMs in younger patients are more likely to lead to severe hemorrhage. It is likely that the pediatric mortality from hemorrhage is related to the higher percentage of AVMs with a deep brain location in children. Fults and Kelly reported mortality in four of six patients who had posterior fossa AVM hemorrhages.21,22 Ondra et al reported a 24-year AVM follow-up assessment in a Finnish population. They reported an annual hemorrhage rate of 4% (for patients with any presentation) and found no difference in hemorrhage rates related to patient age.23 Pollock and colleagues performed a comprehensive analysis of AVM natural history hemorrhage risk.24 In this study of angiographic and anatomical AVM criteria, the factors of a prior history of bleeding, a single draining vein, and diffuse AVM morphology were found to be significantly related to risk for hemorrhage. Based on these data, an individual patient with an AVM had an annual hemorrhage rate between 0.99 and 8.9%.24 Although in that study age was not a significant variable, Mori et al reported a higher mortality from AVM-related hemorrhage in children than in adults.25 Although these hemorrhage rates can be used in a discussion with patients or parents regarding brain AVMs, the actual use of such numbers to predict a risk over time is problematic. A simple risk prediction formula for natural history data that maintains each year of risk as independent can be used.26 This formula is as follows: Thus, for a 15-year-old patient with a 3% annual AVM hemorrhage risk and an expected 62 years of life remaining (according to insurance tables), Although this formula assumes population homogeneity and a uniform natural history among persons of different ages, it is simple and useful for helping patients and parents understand the lifelong risks associated with untreated AVMs (▶ Table 68.1).26 It is important to remember that conservative management is rarely advocated for children with brain AVMs. Gerosa et al warned that the poor results (18 of 56 cases) after conservative management in their series indicated that AVMs should be treated, regardless of the mode of presentation.20 Itoyama et al in their natural history study found better outcomes in children younger than 15 years, emphasizing the great capacity of the pediatric brain to recover from stroke.28 In general, the lifelong risk for hemorrhage mandates therapy in most children because the benefits of AVM removal or obliteration outweigh the therapeutic risks entailed. Although some children may wait for several years until they reach an age more suitable for treatment (e.g., we would rarely perform radiosurgery on a child younger than 2 years), observation usually is recommended only when a multimodality approach would have little chance to provide complete obliteration or improvement of neurologic function. When AVMs are large (Spetzler-Martin grade 5), embolization, radiosurgery, or microsurgery alone, or a combination of these techniques, is not likely to cure significant numbers of patients; it is therefore reasonable to consider conservative management for these children. This is not to say that a large AVM cannot be cured, but the patient must carefully understand the risks involved in this stepwise approach before embarking on a program that may not provide great benefit. We have explored staged-volume radiosurgery for symptomatic patients who have large, critically located AVMs. With this approach, different anatomical components of the AVM are irradiated at 3- to 6-month intervals so that each component receives a high dose; however, the risks can be lessened by reducing regional irradiation and allowing repair of the sublethal effects of radiation. Factors like location in the brain, arterial supply, suitability for embolization leading to volume reduction, lack of relationship of the AVM with critical functional brain, and well-defined nidus must all be understood as well as possible before treatment is selected. Nevertheless, it is often not possible to know what embolization can accomplish without placement of a catheter and a superselective angiographic assessment. Similarly, it is difficult to know how the brain may respond to surgical resection in individual patients. These variables are complex for patients with large AVMs. Although our understanding of the potential risks of these different treatments has improved, dilemmas remain for both patients and physicians in the choice of specific therapies. In the late 1980s, stereotactic radiosurgery became an important therapeutic option for children with brain AVMs.29 Radiosurgery is the single-session delivery of a focused volume of radiation to a defined intracranial target. This surgical option has significantly expanded our options in individual patients. Before stereotactic radiosurgery, the options were surgical resection, embolization, and conservative management.27 Many patients continued to have a patent AVM and a risk for hemorrhage. Radiosurgery provided a new therapeutic approach for the management of deeply located malformations in high-risk locations.30–34 The increased number of radiosurgical facilities worldwide has been followed by increased use of this technology for pediatric AVMs. During a 17-year interval, we performed Gamma Knife radiosurgery in 159 children with brain AVMs: 85 boys and 74 girls with a mean age of 12 years (range, 2 to 17 years). Indications for radiosurgery included all types of clinical presentation, any brain location, and an AVM diameter of less than 3.5 cm (▶ Table 68.2). Some families chose radiosurgery over resection for “operable” AVMs in their desire to avoid craniotomy. Most of these children had small AVMs with a nidus that might have been difficult to locate during surgery (▶ Table 68.3). Most children younger than 13 years receive general anesthesia for radiosurgery. Older children usually require light intravenous sedation. Before radiosurgery, patients and parents speak to the neurosurgeon, radiation oncologist, and radiosurgical nurse regarding the procedure and watch a teaching video tape. Children typically tolerate skull frame fixation well. We use the Leksell model G stereotactic frame (Elekta, Atlanta, GA), which is applied to the outer table of the skull with four-point fixation. The frame is shifted toward the side of the malformation in an attempt to bring the AVM close to the center of the stereotactic space. Patients then undergo contrast-enhanced stereotactic magnetic resonance (MR) imaging with both short TR (time to repetition) imaging and volume acquisition SPGR (spoiled gradient recalled) sequences, used for MR angiographic reconstruction.35 The majority then undergo stereotactic angiography, with all images transferred digitally to a high-speed computer workstation (▶ Fig. 68.1 and ▶ Fig. 68.2). Fig. 68.1 Volume-staged gamma knife radiosurgery plan (16 Gy to the 50% isodose line) for a 12-year-old girl with a thalamic arteriovenous malformation. Axial (upper), coronal (middle), and sagittal (lower) T1-weighted contrast-enhanced and T2-weighted magnetic resonance images showing an anatomical component of the first (blue line) and the second (yellow) volume-staged stereotactic radiosurgery. Fig. 68.2 Upper: Angiograms showing a 12-year-old girl with a thalamic arteriovenous malformation at the time of Gamma Knife radiosurgery. Middle: Angiograms showing total obliteration 38 months after a second volume-staged radiosurgery. Lower: Axial T1-weighted contrast-enhanced and T2-weighted magnetic resonance images showing total obliteration with a slight abnormal area on the T2-weighted image. Radiosurgical dose planning is performed by the neurosurgeon in conjunction with the radiation oncologist and medical physicist. For AVMs with an irregular volume, multiple isocenter plans are created in order to conform the AVM nidus margin to the selected treatment (usually the 50% isodose). The maximal dose is selected based on the weighting of various factors, such as AVM volume, brain location, and pertinent clinical history. With use of the integrated logistic formula (which predicts a 3% risk for permanent radiation-related complications), the appropriate margin dose is selected. We attempt to choose an effective and tolerated dose, balancing the highest obliteration rates achievable with risk factors related to volume and location. Single or multiple isocenter radiation is then performed with the Gamma Knife (Elekta Instrument, Atlanta, GA). As a last step, the stereotactic frame is removed and a local dressing is used to wrap the head. The patient is transferred either to the recovery room (if general anesthesia was required) or back to the hospital room (if local anesthesia was used). All patients are discharged home the next day. Therapeutic anticonvulsant agents are administered to patients with lobar AVMs. A single intravenous dose of methylprednisolone is administered immediately after radiosurgery. For follow-up, we obtain MR images at 6- to 12-month intervals after radiosurgery in order to assess both the vascular and the parenchymal response. An angiogram is requested for all patients beginning 3 years after radiosurgery. When complete obliteration or only an early draining vein is identified on the follow-up angiogram, no further treatment is necessary. For patients who have small residual AVM nidus more than 3 to 3.5 years following radiosurgery, additional treatment is usually required to complete the obliteration. This may involve either microsurgical resection or repeated radiosurgery. In the University of Pittsburgh pediatric series, 87 patients (64%) presented with a hemorrhage, 16 (12%) had headache, and 20 (15%) had experienced prior seizures. Extraocular movement dysfunction was noted in 1 (0.7%) and hand intention tremor in 1 (0.7%). In 10 patients (7%), the diagnosis was made by imaging during the evaluation of a problem unrelated to the AVM. A neurologic deficit was present in 54 patients (40%) at the time of radiosurgery. Prior subtotal surgical resection (range, 1 to 4 operations) had been performed in 22 children (16%). Embolization was performed in 25 patients (19%) in an attempt to reduce the size of the AVM before radiosurgery. The AVMs were located in the cerebral hemispheres in 74 patients, the cerebellum in 5, the corpus callosum in 3, the thalamus in 22, the basal ganglia in 16, the pineal region in 1, and the brainstem in 14. A Spetzler-Martin grade 1 AVM was diagnosed in 3 (2%), grade 2 in 34 (25%), grade 3 in 58 (43%), grade 4 in 17 (13%), grade 5 in 3 (2%), and grade 6 in 23 patients (17%) The mean AVM volume in this series was 2.5 mL (range, 0.1 to 17.5 mL). The median number of isocenters was 4 (range, 1 to 17). The median dose selected to the AVM margin was 20 Gy (range, 15 to 25 Gy), and the median maximum dose was 40 Gy (range, 30 to 50 Gy). In general, an AVM margin dose of 23 to 25 Gy was delivered if possible. This dose range has been associated with the highest obliteration rate in published studies.36 The selected dose is not specifically lower for children than for adults, but attention is paid to AVMs near the hypothalamic–pituitary axis before radiosurgery is chosen as a treatment. We limit irradiation of the pituitary region in an attempt to avoid risks for growth delay. The risk for an AVM hemorrhage is neither increased nor decreased by radiosurgery until complete obliteration has occurred.37 In our study,38 obliteration rates after radiosurgery documented by either angiography or MR imaging criteria were 45%, 64%, 67%, and 72% at 3, 4, 5, and 10 years, respectively, with a median of 71 months of follow-up. The median time until documentation of total obliteration on MR imaging was 37 months. In 81 patients with 4 years or more of follow-up, 57 patients (70%) had total obliteration documented by angiography. Factors associated with a higher rate of documentation of AVM obliteration were smaller AVM target volume, smaller maximum diameter, and larger margin dose. Tanaka et al compared their results after Gamma Knife radiosurgery in 23 children and 76 adults whose mean AVM volumes and margin doses were similar. They found 1-year complete obliteration rates of 74% in children and 45% in adults, and 2-year complete obliteration rates of 95% and 81% in children and adults, respectively.39 These data lend further support to the concept of a higher obliteration rate in children. Pan et al40 reported that AVM obliteration was achieved in 48 (65%) of 74 pediatric patients at 4 years after initial stereotactic radiosurgery. Additional stereotactic radiosurgery, when needed, led to an overall total obliteration rate of 81%. In the previous series, the annual hemorrhage rate for pediatric AVMs after stereotactic radiosurgery ranged from 0.56 to 3.2%.33,40–45 In our study,38 8 patients (6%) had a hemorrhage during the latency interval, and 1 patient died. The rates of AVM hemorrhage after stereotactic radiosurgery were 0%, 1.6%, 2.4%, 5.5%, and 10.0% at 1, 2, 3, 5, and 10 years, respectively. The overall annual hemorrhage rate was 1.8%. Larger-volume AVMs were associated with a significantly higher risk for hemorrhage after stereotactic radiosurgery. No patient bled after documentation of AVM obliteration with either MR imaging or angiography. A larger target volume was associated with higher rate of hemorrhage after stereotactic radiosurgery. Seizures are the second most common presenting symptom of vascular malformations, occurring in 15 to 20% of cases. For children with seizures related to an AVM, the goal of radiosurgery is both AVM obliteration and cessation of seizures. Gerszten et al reviewed our experience in children younger than 18 years who had radiosurgery for AVMs that caused seizures.46 Before radiosurgery, 13 children had seizures; seven had a single seizure and six had multiple seizures. Two children had intractable simple partial seizures. Six patients also had an intraparenchymal hemorrhage. Previous treatments included partial surgical resection (n = 3) and embolization (n = 7). At 1 year after radiosurgery, 11 of the 13 children (85%) with seizures were seizure-free and required no anticonvulsant therapy. Two of these children had a single generalized tonic–clonic seizure after radiosurgery and were found to have subtherapeutic levels of anticonvulsant medication. Both were later successfully weaned from medication. Two patients had a significant decrease in the frequency and severity of simple partial seizures but did not become seizure-free. No difference was found between seizure outcome in children with complete obliteration of their AVM and that in children with residual AVM at follow-up of at least 1 year. It is still not known whether complete removal or obliteration of an AVM leads to consistent control of seizures. Also, no relationship has been established between the occurrence of a preoperative bleed and the likelihood of its causing or aggravating a seizure disorder afterward. In adult radiosurgery series, good seizure control occurs in more than 70% of patients; better results are identified with increasing age and a higher dose of radiation.47 Because the neural tissue surrounding the AVM, not the lesion itself, is the epileptogenic focus, the effect of radiosurgery on the surrounding brain may be related to hemodynamic effects, local irradiation, or some other factor. Some effect of radiosurgery on the brain surrounding the AVM, perhaps improvement of regional brain perfusion, may diminish the neuronal epileptogenic potential. Because the outcome of a child with a seizure disorder may be different from that of an adult with seizures, in terms of both social and intellectual development, an attempt to obtain complete control of seizures without medications is warranted in all patients. Whether surgical extirpation, radiosurgery, or embolization is necessary remains to be determined in individual patients. Children with intractable seizures and an AVM might benefit from inclusion in a preoperative epilepsy protocol in which a combination of approaches could further improve seizure outcome. Because radiosurgery is used for both “resectable” and “unresectable” AVMs, comparisons between outcomes after radiosurgery and those after resection are warranted.48 Knowledge of the anatomical lesion (AVM) and the functional lesion (epileptic focus) likely is probably necessary to obtain an even higher rate of seizure control. Early complications after radiosurgery are rare. Some children may experience brief nausea after anesthesia. All patients have been discharged from the hospital within 24 hours. An immediate postoperative seizure has been rare in our experience since we instituted a protocol of preoperative anticonvulsant agents in patients with subcortical lobar AVMs. As AVM obliteration occurs, parenchymal changes can be identified on serial imaging studies that may or may not be symptomatic, depending on location in the brain.49 Brainstem AVMs are associated with a higher incidence of complications than supratentorial AVMs.50–52 Long-term results are especially important for children and young adults. In our series,38 eight patients (6%) developed adverse radiation effects after stereotactic radiosurgery at a median of 6.6 months (range, 2.8 to 13.2 months). Permanent neurologic deficits developed in two of six patients. A factor associated with a higher rate of symptomatic adverse radiation effects was an AVM location in the brainstem, thalamus, or basal ganglia. Delayed cyst development was diagnosed in one patient (0.7%) with a prior hemorrhage at 56 months after stereotactic radiosurgery, but no additional treatment was required. Yamamoto et al reported the occurrence of cystic parenchymal changes years after AVM obliteration, and we have seen this in two patients in our series.53 We have not noted a delayed radiation-induced malignancy in our experience of 12,000 patients managed for various disorders. Embolization of larger-volume AVMs is sometimes performed before stereotactic radiosurgery in an effort to increase the probability of complete obliteration.21,29,54 The goal of embolization is quite specific when it precedes radiosurgery: permanent volume reduction. Recanalization of a portion of an embolized AVM that lies outside the irradiated volume will place the patient at continued risk for bleeding. Of 45 patients who underwent repeated AVM radiosurgery at our center, 19 (42%) had previously undergone one or more embolization procedures to reduce the volume of the AVM.55 Of these 19 patients, 3 (16%) required second-stage radiosurgery because a portion the embolized AVM recanalized after radiosurgery. Recanalization of AVMs has been observed with both acrylates56,57 and polyvinyl alcohol.58,59 Lasjaunias et al stated that they have not observed recanalization of an AVM at later follow-up when the angiographic findings at 6 to 12 months were strictly normal.60 Embolization also has the potential to divide an AVM into multiple compartments, which can make conformal dose planning difficult.14,29 Multivariate analysis has shown prior embolization to be a negative predictor of successful AVM radiosurgery.61 As a result, we are using embolization before radiosurgery less frequently in the management of larger-volume AVMs. Cavernous malformations can occur sporadically or as an autosomal-dominant familial disorder. They are composed of endothelial cell–lined sinusoids without intervening brain tissue. Although most cavernous malformations are identified in adult patients with minimal or no symptoms when an imaging study is performed, children who have cerebral cavernous malformations usually are symptomatic. Related symptoms can include brain hemorrhage, seizures, and focal neurologic deficits.62 Multiple cavernous malformations usually are seen as part of a familial disorder. In this setting, parents and siblings should undergo imaging studies for both genetic counseling and the identification of specific lesions. It may also be that cavernous malformations develop over time, perhaps first as a microscopic lesion that through repeated microhemorrhage slowly enlarges and subsequently becomes apparent on an imaging study. Mixed cavernous and venous malformations can also occur, with symptoms referable to bleeding or regional venous hypertension.63 Horowitz and Kondziolka identified increasing numbers of malformations within different generations of the same families studied with MR.64 Cavernous malformations that are incidentally recognized have a low annual rate of bleeding. In a large prospective natural history study, Kondziolka et al identified a 0.6% annual hemorrhage rate in patients without a prior symptomatic hemorrhage.65 This finding occurred regardless of age, brain location, or type of presentation (i.e., seizures or headache). However, in patients who had sustained a prior symptomatic hemorrhage, the subsequent annual hemorrhage rate was a magnitude higher, with a 4.5% annual risk for bleeding.65 Thus, children who are found to have a symptomatic cavernous malformation often will require treatment. For those with a single symptomatic lesion, surgical resection should be performed when the malformation is located in a brain region where resection is feasible. If the lesion is contained entirely within the substance of the thalamus or brainstem, resection may not be appropriate. We have used radiosurgery in both children and adults when prior multiple hemorrhages and a deep brain location mandated treatment.66,67 In this setting, radiosurgery was associated with a significant reduction in the hemorrhage rate over time, especially after a 2-year latency interval.68 However, the lack of an imaging test that confirms cure and the potential morbidity of radiosurgery in the brainstem or thalamus argue for its use only in symptomatic patients with malformations in critical locations. To date, 12 children with cavernous malformations have had radiosurgery at our center (mean age, 11 years), with a median of 2 symptomatic hemorrhages per patient (range, 1 to 10).69 Brain locations included the pons/midbrain (n = 6), thalamus,19 and frontal, parietal, occipital, and temporal lobes (1 in each). The mean radiosurgery margin dose was 15.6 Gy. One patient had 3 symptomatic hemorrhages within 2 years after stereotactic radiosurgery. One patient had 1 symptomatic hemorrhage 2 years after stereotactic radiosurgery. The other 8 patients did not have hemorrhage after stereotactic radiosurgery at a median follow-up of 3 years. Venous malformations are congenital anomalies of the venous circulation. Although they are developmental anomalies, they provide drainage of normal brain tissue. Venous malformations usually are located between the deep and superficial venous systems. Common brain locations include the cerebellar hemispheres, brainstem, basal ganglia, frontal lobe, and parietal lobe. Often, venous malformations are diagnosed as an incidental finding on a computed tomographic scan, MR image, or angiogram. There has been no consistent relationship between venous malformations and headaches. Even in patients who present with seizures and are found to have a venous malformation, a causative link is difficult to identify. Some investigators have found that the epileptogenic focus identified by electroencephalography is anatomically separate from the venous malformation.70 Thus, a specific treatment directed at the venous malformation in some patients may not relieve symptoms. In several natural history studies, venous malformations have been associated with extremely low annual hemorrhage rates.71 Although they are occasionally associated with hemorrhage (perhaps when they coexist with a cavernous malformation), resection of a venous malformation usually is not warranted. In the setting of a large hemorrhage in a noncritical location, the venous malformation and hematoma can be removed with relatively low risk. The surgeon must remember that resection of the venous malformation will eliminate the venous drainage from that brain region. If the brain region is critical, then a symptomatic venous infarction may occur. For this reason, many surgeons would evacuate only the hematoma and leave the venous malformation intact. Rothfus et al reported the resection of cerebellar venous malformations in children who had sustained hemorrhages.72 Adverse outcomes due to brain edema have been reported after the use of radiosurgery for venous malformations.73 In general, these functional venous anomalies should be ignored, although they are of clinical and imaging interest. Pearls
68.1 The Natural History of Arteriovenous Malformations in Children
risk for hemorrhage = [1 – (chance of no hemorrhage)]expected years of remaining life
risk for hemorrhage = (1 – 0.97)62 = 85%.27
Age at initial presentation (y)
Estimated years to livea
Lifetime risk for hemorrhage (%)(2%/y)
Lifetime risk for hemorrhage (%)(4%/y)
0
76
79
96
5
71
76
95
10
66
73
93
15
62
71
92
20
57
68
90
aEstimates according to 1992 preliminary life tables prepared by the Metropolitan Life Insurance Company (MetLife), New York City.
68.2 Stereotactic Radiosurgery
Brain location
Number
Percentage (%)
Frontal lobe
21
16
Parietal lobe
14
10
Temporal lobe
20
15
Occipital lobe
19
14
Corpus callosum
5
4
Thalamus
22
16
Basal ganglia
16
12
Pineal region
1
0.7
Cerebellum
5
4
Brainstem
14
14
Spetzler-Martin grade
Number
Percentage (%)
1
3
2.2
2
34
25.2
3
58
43.0
4
17
12.6
5
3
2.1
6
23
17.0
68.2.1 Technique
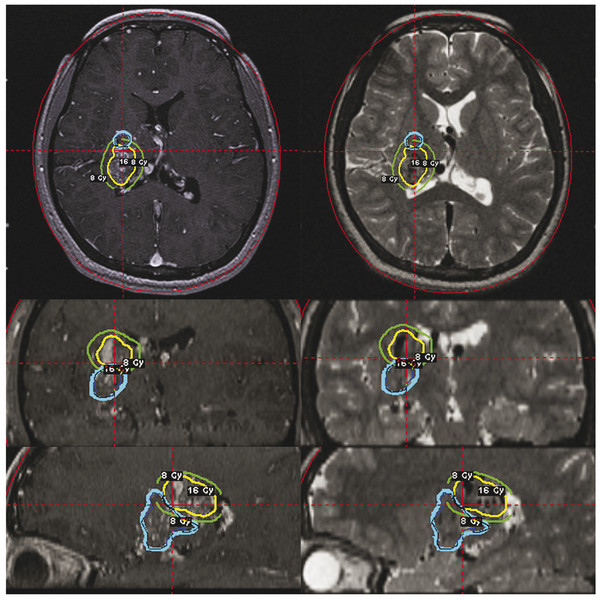
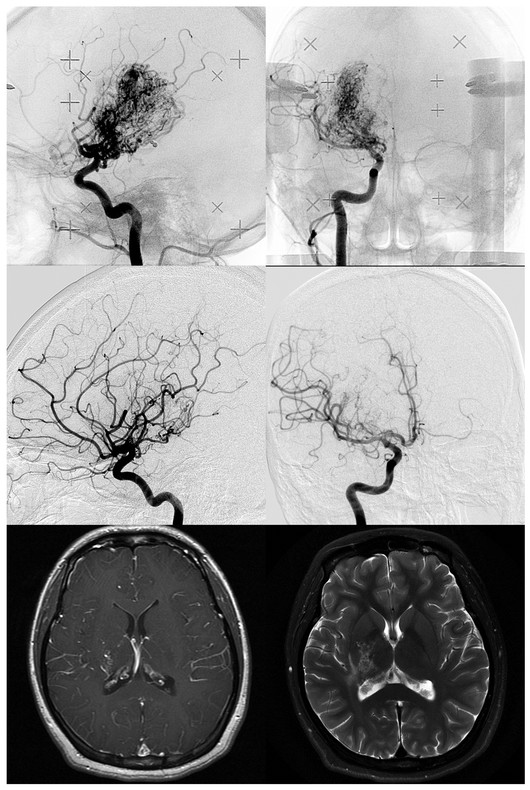
68.3 The University of Pittsburgh Experience
68.3.1 Arteriovenous Malformation Obliteration
68.3.2 Risk for Hemorrhage after Radiosurgery
68.3.3 Results for Arteriovenous Malformations Causing Seizures
68.3.4 Morbidity of Radiosurgery
68.3.5 Role of Embolization before Stereotactic Radiosurgery
68.4 Cavernous Malformations of the Brain
68.5 Venous Malformations (Anomalies)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








