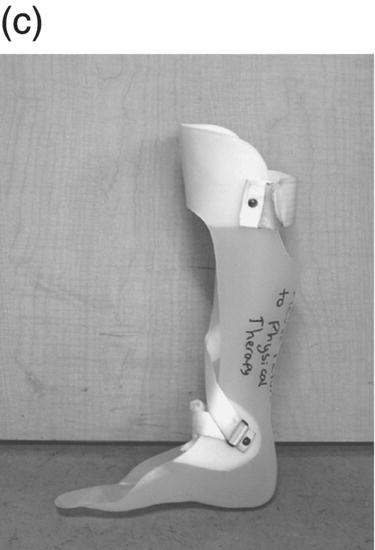
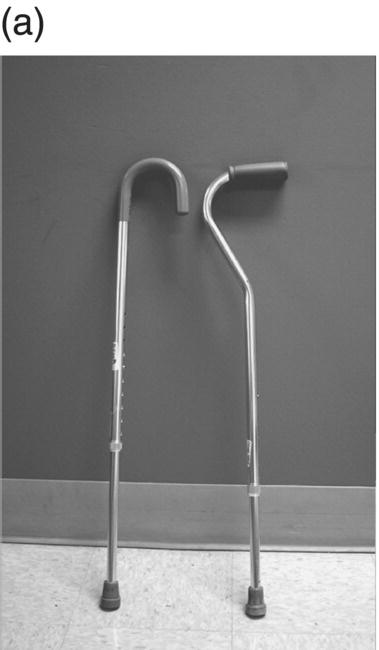
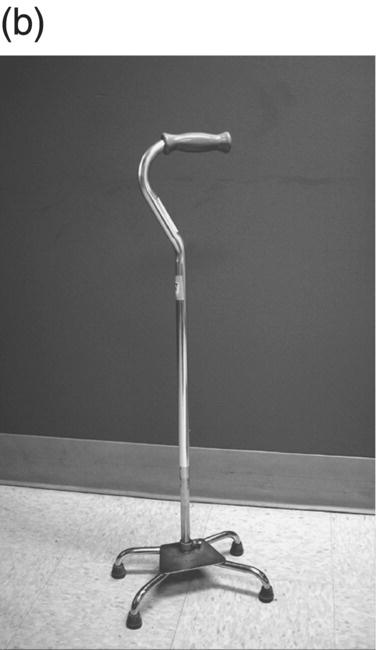
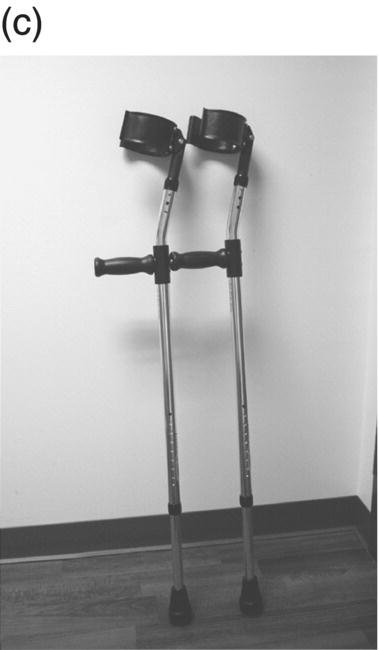
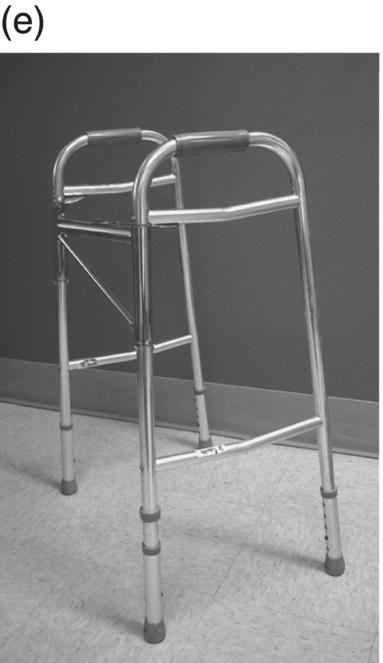
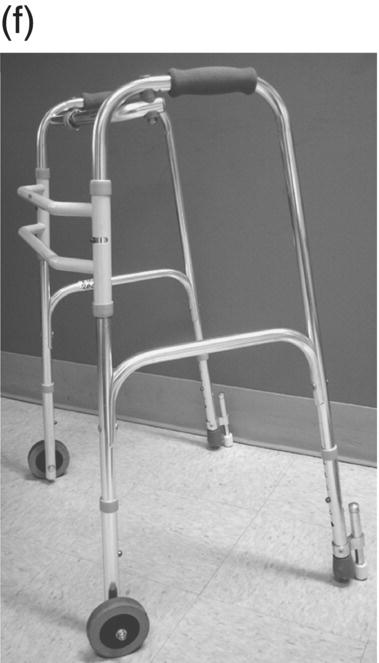
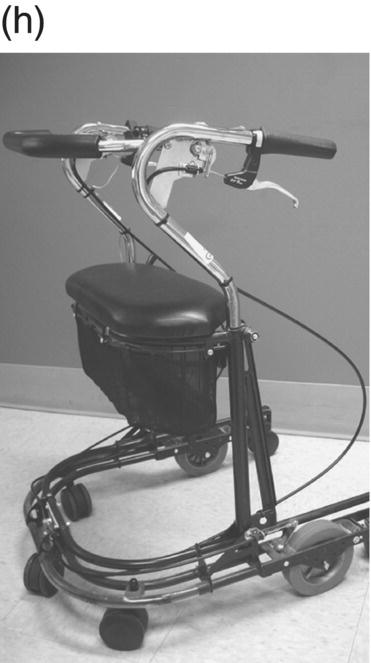
12 Nesanet S. Mitiku1,2, Alexius E. G. Sandoval3, and George H. Kraft4 1 Departments of Rehabilitation Medicine and Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, USA 2 Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, New York, NY, USA 3 Maine Rehabilitation Outpatient Center, Bangor, ME, USA 4 Department of Rehabilitation Medicine and Neurology, Institute for Stem Cell and Regenerative Medicine, University of Washington, Seattle, WA, USA While immunomodulatory and immunosuppressive treatments for multiple sclerosis (MS) are available, none of these treatments provide a cure. Current disease-modifying treatments (DMTs) primarily reduce the frequency of exacerbations and slow disease progression. As a result, the comprehensive management of MS must not only involve optimal DMT selection but also include maximization of current function. Tailoring a program to maximize the current function of an individual is at the heart of MS rehabilitation. Currently, rehabilitation remains the best available way to improve function in MS patients (Kraft 1999). Given the episodic nature of clinical relapses, the uncertain and incomplete extent of neurologic recovery with each relapse, and the gradual progression of disease in a subset of patients, the immediate targets of rehabilitation will change over time. Therefore, the ideal rehabilitation program for any given patient is dynamic so as to best address the evolving disease process, secondary complications, and changing patient goals. Furthermore, even when DMTs fail to adequately restrain disease activity, rehabilitation strategies can improve patient quality of life (QOL). The physical manifestations of MS are manifold, and the observed deficits may result from a combination of sensory, motor, coordination, and cognitive dysfunction. Perhaps nowhere is the confluence of deficits in these systems more prominent than in ambulation. Preservation and maximization of ambulation has many benefits, both in the physical (i.e., mobility, maintenance of bone density, and cardiovascular fitness) and psychological realms. The goal of ambulation is to move from point A to point B in an energetically efficient fashion. Ambulation not only encompasses typical bipedal walking but also includes locomotion via other means such as with a manual or power wheelchair. At a minimum, successful bipedal ambulation requires sufficient antigravity strength to clear the foot during the swing phase of each step together with stability across the ankle, knee, and hip joints. A review of the most common gait abnormalities in MS is provided in the following text, beginning distally with the ankle and progressing proximally to the hip. Insufficient ankle dorsiflexion (ADF) is the most common manifestation of lower extremity pathology in the MS patient. The common abnormal gait patterns associated with this deficit are the foot slap (Figure 12.1a) and steppage gait patterns (Figure 12.1d). In both cases, the usual cause is ADF weakness, but excessive plantar flexion tone or contracture can also produce these gait patterns. When ADF weakness is mild, a foot slap pattern is observed. In contrast, severe ADF weakness will often present with a steppage gait pattern (provided hip flexion strength is preserved). In the former, initial contact will usually occur with the heel, and a loud sound will be heard as the rest of the foot comes down. In the latter, the patient will raise the knees as if marching, and initial contact will be quiet and occur with the front of the foot. Such ADF weakness may not fully manifest on manual motor testing. Indeed, some patients with full strength on manual motor testing may exhibit a foot slap only after walking for some distance. Often, a spouse or family member will endorse loud walking by the patient. The ADF deficit may be uncovered by having the patient walk multiple laps of an office hallway. Suspicion of this type of weakness should be high in a patient who experiences actual or near falls when walking, especially when faced with tasks requiring divided attention. Detection of such weakness is of paramount importance as insufficient foot clearance puts the patient at risk for further injury. Furthermore, there are interventions to compensate for this weakness. Figure 12.1 Common gait abnormalities in MS patients. (a) Foot slap due to mild ADF weakness, (b) knee instability with buckling leading to a fall, (c) Trendelenburg (compensated on the right) finding secondary to hip abduction weakness, and (d) steppage gait with moderate to severe ADF weakness. Each abnormality is aligned with its corresponding phase of the gait cycle. Knee instability secondary to quadriceps weakness (Figure 12.1b) can also prove challenging to the ambulatory MS patient. In order to compensate for this, shortly after the foot makes initial contact with the floor, the patient will snap the knee backward, at times even hyperextending the knee. This maneuver places the ground reaction force closer to the knee axis, increasing stability at that joint. Sometimes, the patient will achieve this rapid extension of the knee by keeping the hand in the ipsilateral pocket and providing a knee extension force by pushing back on the femur with the hand. Chronic forced hyperextension of the knee can lead to permanent ligamentous laxity, increased risk of degenerative changes within the knee joint, and chronic knee pain. Hip girdle weakness can occur in the MS population. Hip abduction weakness produces an excessive pelvic drop during ambulation (Trendelenburg sign, Figure 12.1c) and, when severe, can complicate maintenance of balance. To compensate, a patient may throw the trunk toward the side of weakness during stance phase (compensated Trendelenburg, Figure 12.1c); this strategy produces increased strain on the lumbar spine. In the context of normal gait, hip flexor strength is not overly critical; antigravity strength is all that is required. However, in an MS patient who also has ADF weakness, increased hip flexion strength can help with foot clearance (see steppage gait under Ankle Dorsiflexion). In contrast, hip flexor tightness can be problematic during ambulation as it induces excessive lumbar lordosis, translates the center of mass anteriorly, and, as a result, increases the muscular forces required to stabilize both the knee and the ankle. It is common in individuals with upper motor neuron disease to face barriers to better ambulation as a result of excessive muscular tone. In addition to its biomechanical effects, insufficiently managed spasticity can make walking energetically costly. Plantar flexion spasticity is common in MS. Excessive plantar flexion spasticity can antagonize ADF during the swing phase of ambulation. Additionally, patients with plantar flexion spasticity may have difficulty transferring weight onto the affected leg during the loading response of the gait cycle. Spasticity involving musculature crossing the knee can adversely impact limb clearance during swing phase and limb stability during stance phase. Quadriceps spasticity can prevent adequate knee flexion during swing phase and functionally lengthen the swinging leg, making limb clearance more difficult. Hamstring spasticity may prevent adequate knee extension at the terminal portion of swing phase, leading to early weight transfer onto a leg with a bent knee. Landing with an overly bent knee at loading response will not only shorten the step length but also increase the risk of knee buckling and a subsequent fall (Figure 12.1b). At the hip, patients with excessive adductor tone or spasticity may exhibit a scissoring gait. This gait pattern is characterized by a narrow base of support, placing the patient at increased fall risk. A thorough assessment of spasticity in the context of ambulation is complementary to a manual assessment of spasticity such as with the Modified Ashworth Scale (Bohannon & Smith 1987). The latter will provide a joint-by-joint numerical measure of velocity-dependent tone and can be useful in quantifying treatment response over time. However, gait assessment highlights those muscles contributing most significantly to gait pathology. These muscles will be prime targets for botulinum toxin or phenol injection when normalization of gait is the desired goal. Rehabilitation interventions for the locomotion deficits described earlier fall into six general categories: bracing, functional electrical stimulation (FES), assistive devices (ADs), reduction of spasticity, exercise-based strategies, and pharmacologic intervention. In addition to providing an external limit to movement across joints exhibiting instability, bracing can slightly enhance movement in selected directions. For ADF weakness, an ankle foot orthosis (AFO) can provide adequate foot clearance either by fixing the ankle at an angle that will ensure foot clearance or by providing an assistive force (such as with a spring or its equivalent, Figure 12.2a) to achieve that same foot clearance. If there is additional mediolateral instability of the ankle, the brace should be designed to capture both malleoli so that stability is restored. If there is fluctuation in limb edema or concern about skin integrity, an AFO can be constructed in the context of an orthopedic shoe (i.e., double metal upright AFO, Figure 12.2b). For individuals with mild quadriceps weakness, the ankle joint of the AFO can be placed in slight plantar flexion, or a ground reaction force model (Figure 12.2c) can be used. Patients are often concerned about the comfort, profile, visibility, and/or weight of the brace. AFOs are highly customizable with respect to fit and are available in light materials such as carbon fiber (Figure 12.2d). Most AFOs can fit under a straight-legged pant. Figure 12.2 AFOs. (a) Posterior leaf spring, (b) double metal upright, (c) ground reaction force, and (d) carbon fiber AFOs. For more complex bracing needs (i.e., in the setting of multiple joint instability), the MS patient must be referred to an experienced provider, such as a physiatrist or orthopedist specializing in the management of patients with concomitant joint and upper motor neuron dysfunction. In MS, the lower motor neuron, while intact, is not being driven in a manner to achieve optimal strength. As a result, electrical stimulation of nerves supplying key muscles of ambulation at the appropriate time during the ambulatory cycle can improve ambulatory capacity. Commercially available lower extremity FES devices have been traditionally geared toward assisting ADF through stimulation of the common fibular nerve (Bioness L300 Foot Drop System™, WalkAide®, Odstock Dropped Foot Stimulator®). However, recently, models have become available that have the capacity to address knee instability (Odstock Two Channel Stimulator®, Bioness L300 Plus™) and provide more user customizability as to the target nerve of stimulation (Odstock Two Channel Stimulator®). FES devices are contraindicated in individuals with pacemakers and in those with lower motor neuron injury as a cause of the weakness being corrected (i.e., fibular nerve dysfunction, L4–L5 nerve root injury, or plexopathy). MS patients can have coincident peripheral nerve injuries, radiculopathies, and plexopathies, and these diagnostic entities should be ruled out on physical examination prior to referral for an FES trial. Should ambiguity as to the cause of lower extremity weakness exist, electrodiagnostic testing should be employed for a definitive diagnosis. Once a referral is made for an FES device, the patient will work with an experienced prosthetist or physical therapist on the fitting and programming of the device. Optimization of stimulation and fit may require multiple visits. There are many considerations in deciding between an FES device and a functional brace; in parallel with our caveat earlier on bracing multiple joints, we advise that a referral to an experienced clinician be made before these are prescribed. Handheld ADs increase the patient’s base of support and in so doing improve ambulatory stability. The simplest AD is a single-point cane (Figure 12.3a). A cane should be held opposite to the side of greatest weakness. An extended base cane such as a quad cane (Figure 12.3b) or a hemiwalker can provide even greater unilateral support. In the patient with bilateral weakness or in whom gait instability is not adequately corrected with a unilateral device (i.e., cane), bilateral crutches or a walker should be considered. Forearm crutches (Figure 12.3c) are preferred over axillary crutches (Figure 12.3d) in those who require less weight bearing through the hands. Figure 12.3 ADs for ambulation. (a) Single-point canes, (b) quad cane, (c) forearm crutches, (d) axillary crutches, (e) four-point folding walker, (f) front-wheeled walker, (g) four-wheeled walker with seat and active braking system, and (h) four-wheeled walker with seat and passive braking system (U-Step walker). Walkers are highly customizable with variable height, optional wheels, with or without a seat, with different braking systems, and of different materials of construction. A walker with wheels (Figure 12.3f–h) will require less energy during use as the patient avoids picking up the walker in order to advance forward. A built-in seat (Figure 12.3g and h) provides an instant opportunity for rest; this is particularly important for patients in which fatigability is an issue. A passive braking system in which the default brake setting is on (i.e., the patient depresses the brake lever to walk and releases the lever to stop, Figure 12.3h) is also preferred as this provides the greatest amount of support should sudden imbalance arise. With a passive brake system, the walker will not roll away from the individual trying to avert a fall. Wheelchairs may be prescribed for patients with more severe ambulatory dysfunction. They can be of the manual or power variety. A manual chair is a good option for individuals with moderate trunk control, sufficient upper limb strength and coordination, and adequate cardiovascular fitness. A manual wheelchair can serve as a backup option for MS patients who are only able to ambulate over short distances; they can use the manual chair for outings where longer distances need to be traversed. An electric scooter is another option for the MS patient with limited ambulatory capacity but with good trunk control. Some manual chairs and scooters can be folded or dismantled and transported in the trunk of a car. A power chair is more appropriate for individuals who lack the either the upper extremity (UE) function or cardiopulmonary capacity to propel a manual chair, have reduced trunk control, and/or lack the capacity to perform pressure releases for skin protection. Power chair users must have the cognitive ability to drive a chair safely and demonstrate the physical reliability to drive safely. Power chairs can be driven by hand, mouth, chin, or eye movements. Custom head and trunk positioning devices can be incorporated into power chairs for individuals who require extra support. In either manual or power systems, seat cushions can be designed to provide adequate pressure distribution for the skin so as to minimize the risk of pressure sores. Of note, a patient with MS may need more than one ambulatory aid. A cane may be adequate for short distances, but a walker may be needed for longer distances or at the end of the day when fatigue is worse. Powered mobility with a scooter or power chair may ultimately be required for protracted travel. As previously mentioned, spasticity can add to the energetic cost of ambulation and increase the risk of falls through its biomechanical effects. However, spasticity may also be beneficial; some patients utilize spasticity to facilitate transfers and ambulation. When spasticity serves a useful purpose, overmedication with spasmolytic agents can result in undue weakness. The examiner must identify both the benefits and challenges associated with spasticity in each MS patient. If, on ambulatory examination, the predominant pattern of spasticity is focal in nature, a targeted spasmolytic approach is desirable. Chemodenervation of the specific muscles impeding ambulatory efficiency avoids the systemic side effects of oral medications. Botulinum toxin injections are most effective in combination with an aggressive stretching program, and the initial injection series should be coupled with a supervised stretching and exercise program involving particular attention to the muscles injected. Should the patient require long-term assisted stretching, a caregiver should be identified prior to completion of the supervised therapy program so that the caregiver can be trained directly by the therapist. Phenol neurolysis may be appropriate for individuals in whom spasticity has not responded adequately to botulinum toxin or in whom the single-session upper limit cumulative dose of botulinum toxin is being approached. Phenol is particularly useful for adductor spasticity as targeted neurolysis of the anterior branch of the obturator nerve can produce reduced spastic activity of the entire obturator group. An additional advantage of phenol neurolysis is its long duration of 8–9 months. This is in contrast to botulinum toxin that has a duration of action of 2–3 months. Furthermore, the proceduralist can determine the effect of a phenol injection almost immediately as it works by rapid demyelination and/or destruction of the target nerve. However, the promise of a longer duration of action is tempered by an increased procedure duration (with associated increased patient discomfort) and by a 10% risk of persistent dysesthesias. In individuals where spasticity is widespread, oral spasmolytics or a baclofen pump may be better alternatives. Exercise programs are geared toward improving the patient’s intrinsic abilities. A balanced exercise program involves maintenance or pursuit of adequate flexibility, strengthening, and cardiovascular fitness. Joint range of motion deficits identified on examination should be the focus of targeted stretching programs; this will serve to ameliorate the adverse biomechanical effects of inflexibility. Furthermore, prolonged stretching several times daily is the foundation of a good spasticity management program. Strengthening programs should be designed to correct deficits identified on clinical examination while working toward larger functional goals such as improving balance, increasing independence with transfers, achieving normalization of gait, and increasing stair-climbing tolerance. Cardiovascular fitness ought to be incorporated into every exercise program. Early identification of an enjoyable activity that elevates the patient’s heart rate and can be performed regularly once the individual has graduated from the supervised therapy environment is critical. In some individuals, core temperature elevation with exercise may induce Uhthoff’s phenomenon, a transient episode of neurologic dysfunction secondary to heat-associated conduction block in previously demyelinated segments. Either internal cooling (ingestion of an iced drink) or external cooling strategies (i.e., formal cooling garments, air conditioning, etc.) may be utilized in order to make exercise feasible. Pools selected for aquatic exercise should not be overly heated. Lastly, significant levels of disability do not necessarily preclude exercise. With a little bit of creativity, an exercise program can be created for most individuals. Overall, the benefits of exercise extend beyond physical fitness and increased functional capacity; there is evidence to support a positive impact on both mood and fatigue (Dalgas et al. 2010). So far, we have discussed strategies that alter the biomechanics of walking through focal interventions or through device substitution. In contrast, 4-aminopyridine (4-AP) works to improve walking by addressing one of the hallmarks of MS: demyelination. It improves conduction through demyelinated pathways by influencing voltage-gated potassium channels. While 4-AP has been available in compounded format for some time, its use was limited by its side effect profile, which included a substantial seizure risk. Dalfampridine, a sustained-release version of 4-AP with an improved side effect profile, was shown to improve walking speed in about one-third of patients with MS (Goodman et al. 2009) and was approved by the FDA for this indication in 2010. Increased walking speed has practical benefits (i.e., safely crossing the street, getting to the bathroom in time). While the lower extremities are often more severely affected, the UEs are also at risk in MS, particularly in those with heavy disease involvement of the cervical spine. Particularly disabling is the combination of upper limb intention tremor and dysmetria. Tremor occurs in 20–60% of individuals with MS (Koch et al. 2007). The amplitude of tremor can be minimized through distal weighting of the affected extremity. Deep brain stimulation (DBS) and/or thalamotomy may improve both postural and intentional tremor associated with MS. However, the results do not appear to be sustained, and there is a risk of additional neurologic disability with surgery (Bittar et al. 2005; Hassan et al. 2012). Loss of UE function has greater implications for the performance of activities of daily living (ADLs). ADLs include items such as eating, bathing, grooming, dressing, toileting, transferring, shopping, telephone use, computing, writing, bill paying, and driving. As with lower extremity function, interventions for UE function fall into similar categories: bracing, FES, ADs, reduction of spasticity, and exercise-based strategies. However, the nature of bracing, nerves targeted by FES, goals of the ADs, muscles considered for chemodenervation, and prescribed exercises will be specific to the UE. Ongoing collaboration and communication with an occupational therapist experienced in neurologic disorders will expedite successful individualized solutions for MS patients with UE deficits. In individuals with severely limited UE function, the use of voice-activated environmental control systems will greatly enhance independence and reduce the amount of caregiver supervision required. Alterations to the home according to the needs of the patient can improve QOL greatly. For the wheelchair-enabled individual, the entrance to the home may need to be ramped, doorways widened to accommodate wheelchair width, countertops adjusted to wheelchair height, and bathrooms equipped with a rolling shower chair, no-lip or low-lipped shower, and the appropriate item(s) to ensure safe transfers (grab bars, transfer pole, Hoyer lift). For the ambulatory individual, double handrails should accompany all stairwells. If foot clearance during ambulation is a problem, home floors should be clear of rugs, exposed wires, and other items that could initiate a fall. Organized areas for cooking and grooming can be created so that these processes are as efficient as possible, thus reducing the energetic cost to the individual. Voice-activated controls within the home can increase independence, particularly for those with limited UE function. Enlisting an occupational therapist in the process of determining the most feasible modifications for a particular individual is highly recommended. The modifications that are possible will be guided by the patient’s social, financial, and insurance resources, but additional resources may be obtained through local and national MS support groups. Bladder dysfunction in MS is often characterized by urgency, frequency, and at times incontinences. The most common etiologies are detrusor hyperreflexia and detrusor-sphincter dyssynergia (DSD). In detrusor hyperreflexia, the bladder contracts before it has reached a normal filling volume; the patient is often faced with frequent small-volume voiding. This bladder hyperactivity can be mitigated by the use of antimuscarinic agents such as tolterodine, solifenacin, or oxybutynin; an adequate trial of at least 4 weeks should be given with dose titration during that period. In DSD, coordination between bladder contraction and sphincter opening is compromised; the bladder may at times contract against a closed sphincter producing hesitancy or the urinary sphincter may open in the absence of contraction leading to unanticipated incontinence. Antimuscarinics and catheterization provide the mainstay of DSD treatment; when these strategies are insufficient, referral to a urologist for further workup and treatment options is warranted. Less frequently, a hyporeflexic bladder will be encountered; this may occur in the setting of reduced bladder sensation where the urge to void is not transmitted. As a result, the bladder becomes markedly distended and over time loses its contractility. In men, particularly in those of advancing age, the overall picture may be complicated by urethral obstruction secondary to enlarged prostatic size. A postvoid residual (PVR) provides a quick in-clinic assessment as to whether urinary retention is a problem. The voided volume should also be measured. If the PVR is low and the voided volume is low and the patient has urgency and/or frequency, detrusor hyperreflexia is most likely. If the PVR is low and the voided volume is high, the patient may have a mildly abnormal voiding reflex but retains good detrusor contractility; this patient should be encouraged to void more often in order to avoid overstretching the bladder. If the PVR is high, regardless of whether the voided volume is low or high, retention is present and problematic. This could be due to DSD, reduced detrusor contractility, or sphincter obstruction. Urodynamic testing could distinguish among these possibilities. In men, a trial of an alpha-2-adrenergic agent may be helpful to ascertain the contribution of urethral constriction. If retention persists, a reliable method of emptying the bladder should be employed to avoid increased urinary tract infections, ureteral reflux, and upper tract damage such as hydronephrosis. Effective bladder emptying methods include clean intermittent catheterization and use of an indwelling catheter. Like bladder dysfunction, bowel dysfunction is common in MS and most frequently presents as constipation. In addition to disease-related reduction in bowel motility, extrinsic factors such as low fluid intake, low fiber intake, and medication side effects (i.e., anticholinergic medications, opiate medications) contribute to the overall picture. A thorough investigation of the patient’s current bowel pattern (frequency, regularity, and consistency), dietary practices, and current medications should be undertaken prior to pharmacologic management of bowel symptoms. Modification of dietary practices or substitution of anticholinergic medications may provide an adequate solution. Increased physical activity can also improve bowel motility. If pharmacologic agents are needed, stool softeners (i.e., docusate), bulking agents (i.e., psyllium, calcium polycarbophil), or motility agents (i.e., polyethylene glycol, magnesium hydroxide, magnesium citrate, bisacodyl) can be selected as appropriate for the patient’s bowel history. The ideal bowel program is arrived at methodologically and is regular in practice as well as regular in clinical result.
Rehabilitation
Introduction
Physical rehabilitation
Ambulation: Principles and perturbations
Ankle dorsiflexion
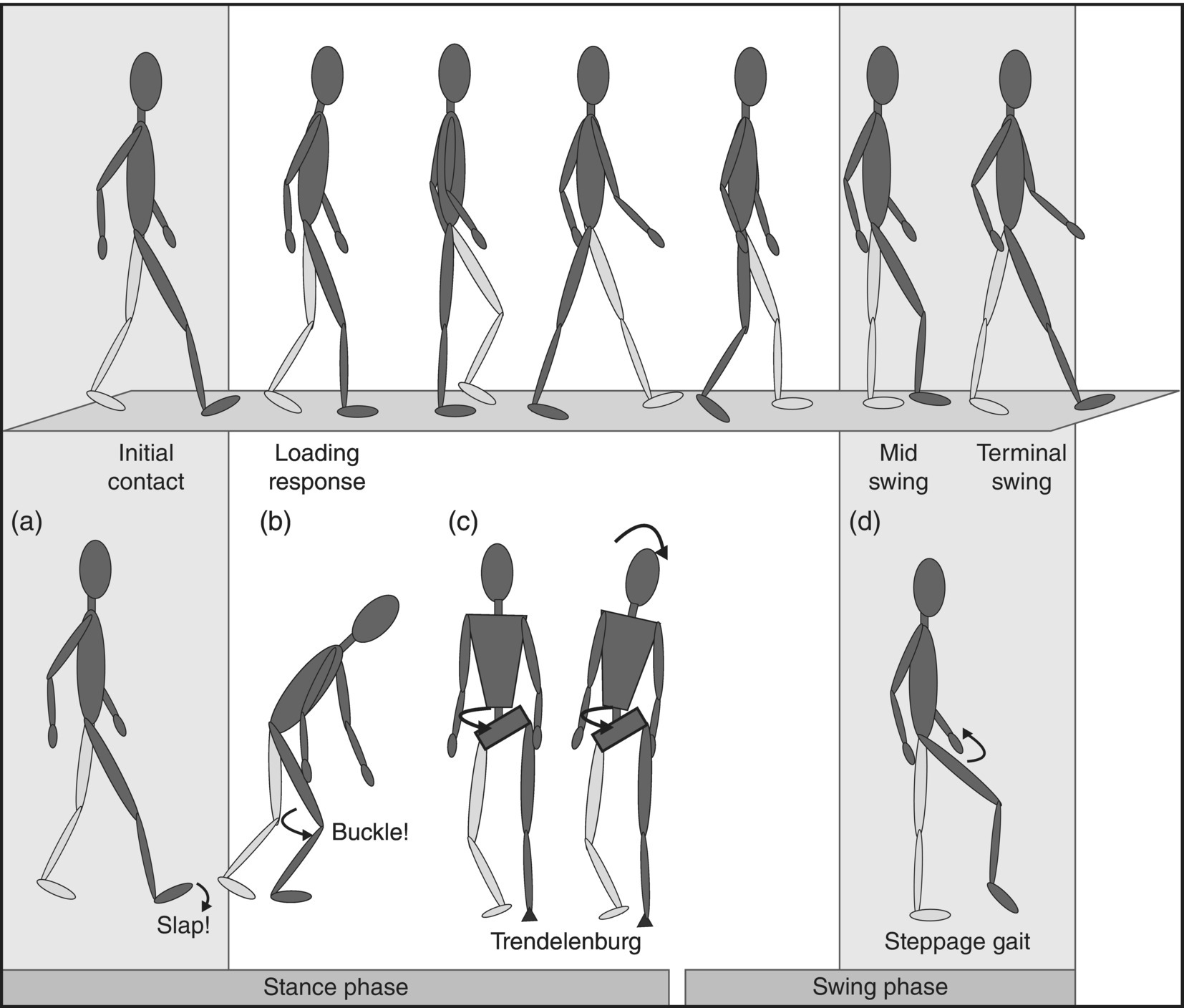
Knee control
Hip girdle stability
The influence of spasticity
Improving ambulation
Bracing



Functional electrical stimulation
Assistive devices


Reduction of spasticity
Exercise-based programs
A pharmacologic approach
Upper extremity function
Home modifications
Organ rehabilitation: Bladder and bowel management
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



