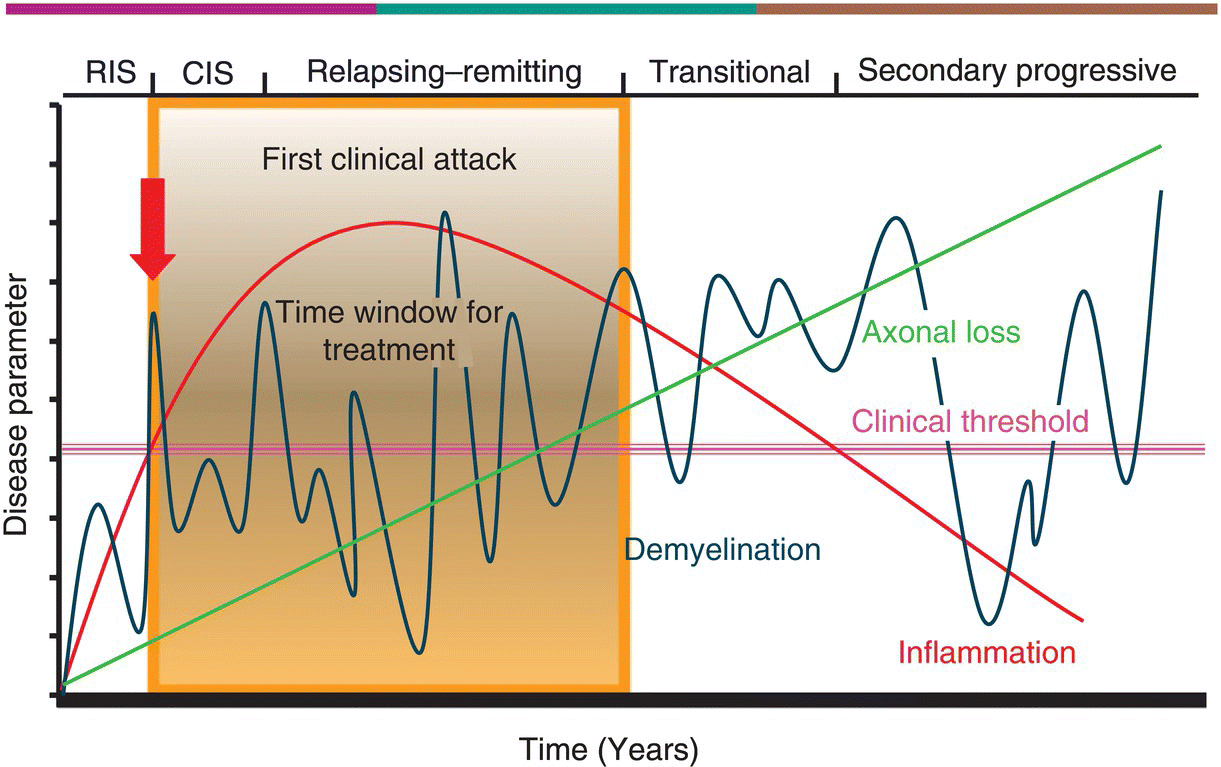
5 Mohsen Khoshnam and Mark Freedman Multiple Sclerosis Research Unit, University of Ottawa, Ottawa, Ontario, Canada Multiple sclerosis (MS) starts out as a relapsing–remitting course in more than 80% of individuals and continues for almost two decades before yielding to secondary progressive (SP) MS. Most of the current disease-specific therapeutics in MS are approved for the relapsing phase with some modest but validated effects: they reduce attacks and MRI activity and slow the development of Expanded Disability Status Scale (EDSS) progression. Studies have shown that the earlier in the course of disease these agents are given, the better the chances of delaying disease progression. For nearly two decades, the mainstay of treatment was one of two therapies: interferon-β (IFN-β) or glatiramer acetate (GA). We are now moving into an era where additional choices exist, but choosing the best option for a given patient is the challenge. As data accumulate, we are beginning to see reasons for moving away from an empiric one shoe fits all approach to therapy to a more personalized patient-specific approach. Disease-modifying drugs (DMD) started with the introduction of IFN-β-1b for the treatment of relapsing MS in 1993, followed by another IFN-β preparation IFN-β-1a (subcutaneous (SC) and IM) and by GA. These have been considered first-line agents in most countries. Mitoxantrone, a well-known chemotherapeutic agent, also attained a level of evidence that led to its approval for treating some MS patients showing signs of disease progression despite treatment with the first-line agents. Natalizumab was the first of a new wave of medications to be approved but was withdrawn from the market by the manufacturer because of emergence of PML and then reintroduced in 2006 with a risk management strategy. In 2010, fingolimod became the first oral DMD available, but there is inconsistency across the world as to whether it too should be considered second line, owing to a different toxicity profile, or first line, as is its current status in the USA—with a new safety recommendation from the FDA. Although the exact mechanism(s) of action (MoA) of all the agents is not well understood, their role in MS disease control is believed to be primarily exerted by immunomodulation (though some may have inherent immunosuppressive capabilities), in that they affect immune system processes that are felt to contribute to the inflammatory events that cause damage to the CNS in MS as discussed in Chapter 2. Table 5.1 shows data on current DMD with their postulated MoA. Table 5.1 Current DMD Administration, MoA, Adverse Reactions, and Pivotal Study No single drug has proven yet to be universally efficacious in abrogating all measures of disease activity in MS and halting the disability progress. Furthermore, there are limited comparative data among current MS DMD to guide clinicians to choose the best option as an initial therapy. Nevertheless, data derived from a few active comparator studies evaluating the efficacy of DMD revealed that high-dose, high-frequency IFN-β preparations are superior to low-dose once weekly IFN-β, but were not shown to be superior to GA. Comparison of fingolimod to low-dose IFN-β-1a IM weekly injection demonstrated superiority of efficacy of fingolimod, albeit with a different and occasional serious side effect profile. We use a classification of these agents into several groups based on risk benefit, yielding to the designation of first line, second line, or further. The first-line drugs are those with long postmarketing safety records and consist of the various IFN-β preparations and GA. In our practice, the second-line group currently consists of natalizumab, mitoxantrone, and fingolimod (though regulatory approval of fingolimod and natalizumab allows for first-line use in the USA), which are agents that might be more potent than first-line drugs but carry a higher risk of major side effects. All are associated with rare but life-threatening side effects. It is proposed that MS is essentially a two-stage disease: an early inflammatory stage, characterized by focal inflammation both in white and gray matter, and a later neurodegenerative stage, which is founded on the neuroaxonal damage caused by the inflammatory process that has already attained a level that is irreversible and progressive and cannot be adequately repaired. In fact, recent evidence indicates that attacks in the first 1 or 2 years may have a major influence on later stage progression, while attacks occurring later may have less influence on disease progression. This suggests that an important early window of opportunity is present for initiation of disease-specific treatment that can minimize the inflammatory-induced damage, as is the goal of all DMD, accomplished through modulation of inflammatory events (manifest as clinical relapse and MRI activity). Effective early therapeutic intervention appears, therefore, key to diminishing the pathological process and preventing or slowing disability progression. Figure 5.1 depicts the window of opportunity. Figure 5.1 MS: Pathology versus clinical course of the disease. Several studies have shown that a poorer prognosis can be anticipated in cases with some of the following characteristics: male gender; a later age at onset; motor, cerebellar, and sphincter involvement at onset; a progressive course at onset; a short interattack interval; a high number of early attacks; and a relevant early residual neurologic deficit. Some paraclinical measures might also confer prognostic considerations such as MRI, cerebrospinal fluid (CSF) analysis, and evoked potentials. These tend to not only predict the early conversion to a relapsing course in CIS patients but also indicate which patients may have an earlier progression (e.g., MRI). In addition to giving us a prognosis, some of these features might also help to place a particular patient into the window of therapy by indicating whether it is early, warranting a safe but mild treatment, or late, where more aggressive therapy may be more effective. The pivotal studies that established current therapies, together with postmarketing monitoring of their efficacy in last two decades, have revealed that they are not curative but can offer partial efficacy in terms of disease control with a variable degree of response among patients. There is no indication as yet of an a priori profile in a patient’s biologic or clinical characteristics to guide us to the best choice of therapy that will produce the optimal response in a given patient, but recently, researchers have suggested some biological markers that might predict treatment response or be used for monitoring treatment in MS patients in the future. Overall, the research offers tantalizing data, and we anxiously await the results of larger studies on bigger cohorts of patients for maturation of the novel idea of biomarkers in MS treatment optimization, and until then, we must rely on common markers of disease activity (relapse, disability progression, and MRI activity) in our clinical practice. The major factors that affect our decision-making for choosing an option in MS treatment are: After starting patients on DMD, we enter into a dynamic and sometimes challenging phase of treatment monitoring. The main purposes of this phase are to determine a patient’s adherence to the regimen, which is a prerequisite for effectiveness, and to assess disease response. Patient adherence involves the combination of patient interest and enthusiasm in maintaining therapy, tolerance to side effects, and the regimen schedule. Adherence can be maximized through educational discussion in a realistic discussion about the expected efficacy of treatment and its usual side effects. Furthermore, adherence is aided through methods and instruments to improve injection techniques and lower the rate of injection site problems. Systemic side effects of IFN-β, such as flu-like symptoms, can be diminished by proper timing for injection according to a patient’s activity and premedication. Detecting drug side effects is partly based on a patient’s knowledge of symptoms, and this in turn requires up-front education about the medication and how it should be used. Table 5.2 summarizes some laboratory tests that should be considered, depending on the individual, before starting the relevant therapy as part of premedication evaluation and after starting medication for side effect monitoring. Table 5.2 Pre- and Postmedication Laboratory Tests CBC/diff., complete blood count and differential; Cr., creatinine; LFT, liver function tests; LVEF, left ventricular ejection fraction; TFT, thyroid function tests. Disease response monitoring is by far the more challenging part of management, as there is no standardized evidence-based protocol for this and we rely mostly on treatment recommendations by consensus opinion. Unlike clinical trials, there is no placebo group with which to compare a patient’s response to therapy; thus, the best practice is to compare the patient’s clinical state to the pretreatment period. This is even more challenging in CIS patients, since we do not have any pretreatment phase of activity. Typically, we would monitor three parameters: relapse, disease progression (EDSS), and MRI, in addition to insuring that each patient is tolerant of the medication and not showing any signs of toxicity. Relapse parameters that concern us as to the level of disease activity are the rate, severity, and degree of recovery of neurological dysfunctions following each attack. The Kurtzke EDSS is the most common measurement of disease progression assessment that has been used both in the clinic and in clinical trials with MS patients. Any increase from the baseline could indicate progression of disease or residual effects from relapses that should be interpreted based on the baseline score and time course of disability establishment. A significant and sustained increase in EDSS following a relapse developing in a short period of time could indicate a more severe inflammatory process. EDSS increments sustained for more than 6 months are usually considered indicative of disability progression. MRI measures of disease activity such as contrast-enhancing lesion(s) (CEL), new or enlarging T2-hyperintense lesion(s), T1-hypointense lesion(s) (or black holes), and atrophy have all been examined, but new T2 lesions +/− CEL are the most consistent and validated measures of disease activity. MRI has already been utilized as a surrogate marker of treatment efficacy in drug trials. A meta-analysis of 23 randomized placebo-controlled trials in RRMS, involving a total of 6591 patients, identified a strong correlation between the effect of treatment on relapses and number of MRI lesions measured. More than 80% of the variance in treatment effects on relapses was explained by the variance in MRI measures. Contrast enhancement and new or newly enlarging lesions identified with T2 have greater utility in evaluating effectiveness of treatment, and MRI metrics (atrophy and T1 black holes) have weak predictive value for future disability and correlate poorly with T2 lesion burden in progressive disease. None of the current guidelines for monitoring therapy have been standardized in large cohort of patients in long prospective studies but are based on clinical and MRI data that indicates inflammatory or degenerative activity of MS in patients, reflecting the temporary or permanent damage in myelin and neuroaxonal tissues. One practical recommendation is a simplified version of the Rio score instrument, a tool that uses the same clinical and MRI measures over the first year of treatment to predict response to therapy. The Rio scoring system was found to be useful for predicting response to SC IFN-β-1a among 373 patients with RRMS who were followed for 4 years in the PRISMS study. The patients were classified at 1 year using the Rio criteria, which determine response based on a score of 0 (responsive) or a score of 1–3 (unresponsive) when 1 point is assigned to each of the following: After 1 year of treatment, the patients were classified using a simplified Rio scoring system (EDSS not included) in which 1 point is assigned to the presence of ≥2 active T2 lesions between months 6 and 12 and relapse is scored as follows: After 1 year, 29.9% of patients were Rio responders, and 37.3% were responders according to the simplified score. Both the Rio score and the simplified Rio score significantly predicted subsequent relapse frequency (p < 0.001 for each) as well as disease progression (p = 0.03 and p = 0.002, respectively). Such a practical guideline can be applied in clinical practice to monitor the response to therapy and determine the status of patient as a responder or nonresponder for planning the future of management.
Relapsing MS: Disease Staging and Therapeutic Algorithms
Introduction
Drug
Route and Dosage
MoA
Risks and Adverse Reactions
Pivotal Study
IFN-β-1b
SC, IFN-β-1b 8 MIU every other day
Reduction in T-cell activation, induction of cytokine shift in favor of anti-inflammatory effect, prevention of T-cell adhesion and extravasation across the blood–brain barrier (BBB), induction of T-regulatory cells, apoptosis of autoreactive T cells, neurotrophic factor expression, antiviral effects
Lymphopenia, injection site reaction, asthenia, elevated liver enzymes, flu-like symptoms, depression, suicidal ideation, and injection site necrosis
IFN-β MSSG 1993
IFN-γ antagonism
IFN-β-1a
SC, 22 µg or 44 µg three times per week
Same as INF-β-1b
Same as INF-β-1b
PRISMS 1998
IFN-β-1a
IM, 30 µg weekly
Same as INF-β-1b
Same as INF-β-1b, flu-like symptoms more pronounced and lingering
MSCRG 1996
GA
SC, 20 mg daily
Induction of regulatory T cell by a shift from Th1 to Th2 and Th3 type; dose-dependent inhibition of MBP-specific T-cell responses; GA-specific Th2-type cell migration through the BBB; cross-reactivity of GA-induced T cells with MBP, MOG, and PLP; bystander suppression; neuroprotection due to increased production of brain-derived neurotrophic factor (BDNF) by GA-specific T cells
Approximately 15% of patients experience a self-limited, postinjection systemic reaction characterized by chest tightness, flushing, anxiety, dyspnea, and palpitations; minor skin site reactions; lipoatrophy
Cop 1 MSSG 1995
Natalizumab
IV, 300 mg every 4 weeks
Antagonist of VLA-4 inhibits the binding of leukocytes to vascular cell adhesion molecules (VCAM)-1 on the BBB and prevents trafficking into the CNS
Most commonly, headache, fatigue, arthralgia, urinary tract infection, lower respiratory tracts infections, gastroenteritis, vaginitis, extremity pain, diarrhea, and hypersensitivity reactions (mainly rash and urticaria); rare but serious adverse effects; cerebral lymphoma, liver toxicity, melanoma, and PML
AFFIRM 2006
Mitoxantrone
IV, 12 mg/m2 body surface every 1–3 months to a maximum of 100–120 mg/m2
Intercalates with DNA, causing single- and double-stranded breaks, and inhibits DNA repair via inhibition of topoisomerase II, inducing immunosuppressive and immunomodulatory properties
Treatment-related leukemia, congestive heart failure due to cardiotoxicity, and infertility
MIMS 2002
Fingolimod
Oral, 0.5 mg daily
Modulation of sphingosine-1-phosphate (S1P) receptors in lymphoid tissues trapping autoimmune cells in lymph nodes
Nasopharyngitis, dyspnea, headache, diarrhea, nausea, and asymptomatic elevations of liver enzymes, rarely macular edema and treatment-induced bradycardia, most commonly seen with the first dose and cardiac arrythmia
FREEDOMS 2010
Window
Prognostic factors
Therapy
Agent
IFN-β
GA
Natalizumab
Fingolimod
Mitoxantrone
Prior to initiation
CBC/diff., LFT, and TFT
N/A
Chest X-ray, HIV, CBC, serum electrolytes, urea, Cr., LFT, serum protein and albumin, beta-HCG in women of reproductive age, TFT, screening of immune deficiency by counting CD4/CD8 cells, anti-JCV Ab titer
CBC, LFT, serum cholesterol level, ECG and retinal examination in diabetics and those with history of uveitis, anti-VZV antibody titer to ensure the immunity
CBC/diff., cr, LFT, beta-HCG in women of reproductive age, LV gated scan or echocardiography for study of LV (LVEF must be >50%)
Monitoring post dosing
CBC/diff., LFT monthly for 6 months and then every 6 months plus TFT
N/A
CBC/diff., LFT, and serum proteins will be done 1 month after the first infusion and then every 3 months, antinatalizumab Ab titer after first or second infusion, anti- JCV Ab titer reanalysis if negative at baseline
CBC and LFT are done in 3 months time for the first year after initiation of therapy with a retinal exam for all treated patients in between 3 and 6 month; serum cholesterol level is done yearly
After each infusion, CBC/diff., Cr., LFT, and beta-HCG (in women) will be tested. After the sixth dose, another LV gated scan or echocardiography is done (LVEF must be >50%), and then yearly CBC/diff. is done for the rest of patient’s life
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



