CHAPTER 380 Revascularization Techniques for Complex Aneurysms and Skull Base Tumors
After its introduction in 1967 by Donaghy and Yasargil,1 the technique of extracranial-to-intracranial (EC/IC) arterial bypass was envisioned as a surgical strategy to prevent ischemic stroke in patients with carotid and intracranial arterial occlusion. Although the results of the EC-IC Bypass Study Group failed to establish that bypass reduced the risk for stroke,2 the use of bypass coupled with parent artery occlusion provides a means to treat complex unclippable and uncoilable aneurysms and cranial base tumors involving major cerebral arteries that could not be adequately treated otherwise without risking ischemic injury. These techniques for surgical revascularization in the treatment of aneurysms and tumors have been pioneered and later refined by Sundt and colleagues,3,4 Ausman and coworkers,5 Ito,6 Peerless and Hampf,7 Lawton and colleagues,8 Sen and Sekhar,9,10 Martin and colleagues,11–14 and others.
Revascularization For The Treatment Of Aneurysms
The optimal treatment of intracranial aneurysms is surgical clipping or endovascular coiling. However, clipping or coiling of complex, giant, and fusiform aneurysms, which incorporate the parent artery or adjacent arterial branches into the aneurysm base or fundus, may be impossible.3–5,7,8,15–19 Calcification or atherosclerotic thickening of the aneurysm neck or the parent artery can make clipping dangerous or impossible. Additionally, recurrent aneurysms after endovascular coil embolization may be unclippable because of the stenting or obstructing effect of the coils on the aneurysm neck.20
Hypothermic circulatory arrest was first used as a surgical adjunct for complex, giant intracranial aneurysms in the 1960s and was refined dramatically in the 1980s.21–24 This technique has been particularly useful in treating giant posterior circulation aneurysms by allowing the aneurysm to collapse, thereby permitting the surgeon to then reconstruct the aneurysm neck with clips. However, hypothermic circulatory arrest is a complex procedure and carries its own risks.22,23 Even with this method it may be impossible to reconstruct the aneurysm neck in a way that preserves the parent artery or branching vessels, or both. Therefore, parent artery occlusion or aneurysm trapping and distal bypass may often be a superior alternative.
Revascularization For The Treatment Of Skull Base Tumors
The petrous and cavernous internal carotid artery (ICA) can frequently be involved by anterior and middle skull base tumors. The most common tumor types that present this surgical challenge are meningiomas, schwannomas, pituitary adenomas, angiofibromas, and chordomas. Because of the benign nature of these tumor types, the carotid is most frequently partially or completely encased by tumor rather than invaded.25 However, some tumors such as meningiomas, particularly recurrent ones, may be very densely adherent to the ICA. In such cases, complete resection is not possible unless the carotid artery is resected along with the tumor, with or without a revascularization procedure. Malignant skull base and head and neck tumors are more likely to invade the vessel itself. In these cases, radical oncologic resection, if that is an appropriate goal, may require carotid occlusion and resection.26,27
The decision to remove the carotid during resection of skull base tumors is controversial. Some authors advocate ICA resection for cavernous sinus and skull base meningiomas.10,28 However, with benign tumors, many experts prefer to aggressively remove the tumor up to the artery but leave the artery intact, even if this means leaving a small residual of tumor adherent to the vessel.25,29 In the case of benign tumors, representative contemporary series with and without ICA resection show only a small difference in rates of gross total tumor resection and recurrence.10,28,29 Given the efficacy of contemporary stereotactic radiosurgery and fractionated stereotactic radiotherapy, we rarely advocate carotid artery occlusion or excision for the treatment of skull base tumors.
Opposition to ICA resection is fueled by the morbidity associated with such procedures and the treatment alternatives now available for benign lesions. All series of ICA resection and bypass have reported complications associated with these bypasses, particularly with the use of saphenous vein grafts.8,10 The rate of ischemic complications and graft occlusion typically exceeds 10%. Additionally, stereotactic radiosurgery is an effective strategy to treat tumors of the skull base with less likelihood of injury to neurovascular structures. Benign tumors, such as meningiomas and schwannomas, are often very sensitive to radiation treatment.30–33 This argues against aggressive surgical resection with carotid sacrifice for these lesions and supports more conservative surgical debulking and leaving tumor adherent to sensitive structures. There is therefore a growing consensus that the carotid artery should not be resected for most nonmalignant tumors.25,28 Lawton and Spetzler, in a review of ICA sacrifice for the resection of skull base tumors, indicated that they sacrificed the carotid artery and performed revascularization in only 10 of more than 300 patients with anterior skull base tumors.25
The poor prognosis associated with malignant head and neck cancers that can involve the anterior skull base has led some surgeons to consider radical tumor removal with ICA sacrifice.10,26,27 If the carotid—or another major intracranial artery—is the only structure that stands in the way of a complete and potentially curative resection, ICA sacrifice with bypass should be considered. The high rate of morbidity related to carotid artery sacrifice alone, without bypass, and the modest rate of morbidity associated with balloon test occlusion suggest that selective revascularization should be considered when arterial resection is deemed essential to achieve an oncologically meaningful resection.26,27 Preoperative evaluation by angiography and balloon test occlusion (with or without hypotensive provocative testing or cerebral blood flow measurements) may aid in the identification of patients who can tolerate carotid sacrifice. Finally, if the carotid has already ruptured or if rupture appears imminent because of tumor invasion, radionecrosis, or previous surgical arterial injury, ICA resection with revascularization should be strongly considered.
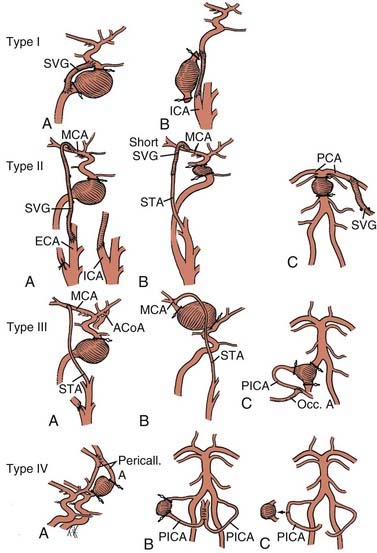
If bypass is deemed necessary in the management of a skull base tumor, the revascularization procedure can be done as a separate, preliminary staged procedure or at the same time as the tumor resection. In general, three types of revascularization can be performed in these situations: the carotid artery can be replaced with an interposition saphenous vein graft (type I), a saphenous vein graft can be placed from the ICA or external carotid artery (ECA) to the middle cerebral artery (MCA) or other intracranial vessel (type II), or the superficial temporal artery (STA) can be anastomosed to the MCA (type III) (Fig. 380-1).
When Is The Collateral Circulation Inadequate And Bypass Necessary?
Anterior Circulation
Elective occlusion or resection of the ICA has been associated with complications in 30% to 45% of cases, but experience with carotid test occlusion suggests that 80% to 90% of patients tolerate ICA occlusion (at least acutely).34,35 Many clinicians advocate a selective approach to surgical revascularization when therapeutic ICA occlusion is planned.36 This approach involves angiographic evaluation of the competence of the circle of Willis and balloon test occlusion coupled with measurement of cerebral blood flow or a hypotensive challenge to identify patients with inadequate or marginal collateral circulation who require bypass in conjunction with parent artery occlusion. Others argue for a universal approach and advocate bypass for all patients who undergo ICA occlusion.8 This strategy is intended to avoid the risk associated with the balloon test occlusion itself, minimize the risk for delayed or chronic cerebral ischemia, and avoid inducing new aneurysms on collateral vessels. Complications related to the balloon test occlusion procedure alone can occur in approximately 3% of patients.37 However, given the relative safety of balloon test occlusion with contemporary endovascular technique, most centers reserve the use of bypass for patients with demonstrably insufficient or marginal collateral circulation in the ICA territory.35,38,39 It should be noted, however, that even if a patient passes a balloon test occlusion, there remains up to a 20% chance of stroke with complete occlusion without a bypass.40
Posterior Circulation
Unclippable and uncoilable posterior circulation aneurysms may require vertebral artery occlusion. In most patients, unilateral vertebral artery occlusion is well tolerated when the contralateral vertebral artery is not hypoplastic and does not terminate in the posterior inferior cerebellar artery (PICA). Bilateral vertebral artery or basilar artery occlusion is associated with a much higher risk for ischemia and should be considered only if blood flow through both posterior communicating arteries is sufficient (>1 mm).18 However, the posterior cerebral artery (PCA) appears to have relatively good collateral potential from leptomeningeal interconnections with the distal temporal and occipital MCA branches. Proximal occlusion of this artery for giant or fusiform arteries is usually well tolerated, with an ischemic deficit (hemianopia) developing in only a few patients.16
Surgical Technique
Intraoperative Monitoring and Management
The patient is allowed to become mildly hypothermic (34°C to 36°C).41 Metabolic suppressive therapy provides cerebral protection during the period of cerebral artery occlusion while the bypass anastomosis is performed.41 We typically use thiopental rather than propofol or etomidate. The efficacy of barbiturates for cerebral protection during transient focal ischemia is supported most strongly by laboratory and clinical evidence.8,41,42 The barbiturates are administered to induce electroencephalographic burst suppression until the anastomosis is complete and the recipient vessels are deoccluded.
Types of Revascularization Procedures
Strategies for cerebral revascularization after parent artery occlusion (see Fig. 380-1) include the use of various vessels as the bypass conduit (e.g., STA, occipital artery, or long or short saphenous vein or radial artery) and the selection of various intracranial arterial sites for the distal anastomosis. These variations can be classified into four types of bypass.12–14
Type I Bypasses—Interposition Vein Grafts
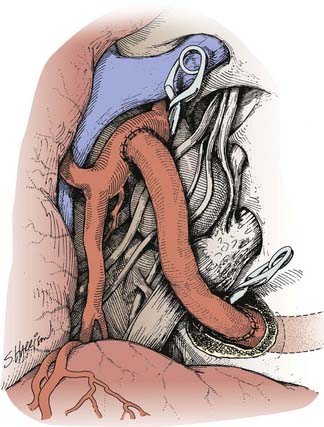
A type I bypass involves an interposition graft from the parent artery proximal to the site of the occlusion to the point immediately distal to the parent artery (Figs. 380-2 and 380-3). The primary example of this type of revascularization is the purely intracranial petrous carotid–to–supraclinoid carotid saphenous vein interposition graft. It is used primarily to reconstruct the carotid artery when it must be resected to remove skull base tumors and to treat giant intracavernous carotid aneurysms.10,43–45 This graft has the disadvantages of being technically complex, requiring a lengthy procedure, and most importantly, necessitating a prolonged period of ICA occlusion. It is associated with a significant complication rate related to graft occlusion and perioperative ischemic brain injury.8,10 A comprehensive description of this procedure is not included because we prefer to use a type II procedure for these indications. Readers are referred to technical descriptions elsewhere.44,45 Of note, carotid artery replacement with a saphenous vein or other interposition graft of this type can be used effectively to reconstruct the cervical carotid artery, below the skull base, if required for the treatment of extracranial carotid aneurysms or neck tumors.27,46–48
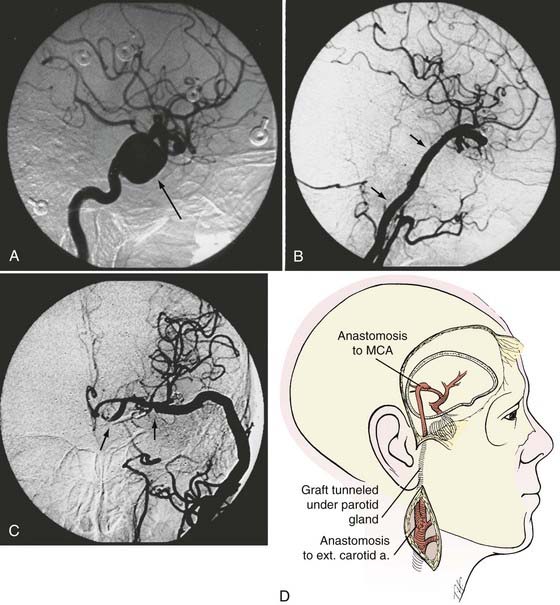
Type II Bypasses—Extracranial-to-Intracranial Bypass with a Saphenous Vein Graft or Radial Artery Graft
A type II bypass consists of a saphenous vein interposition graft between the extracranial carotid artery and a major intracranial branch vessel (Figs. 380-4 and 380-5).49 This procedure is used when a major arterial trunk must be occluded to treat a tumor or giant aneurysm and the distal collateral circulation is grossly inadequate (as evidenced by the absence of communicating arteries seen angiographically or by the rapid onset of a deficit during balloon test occlusion).4,8 In such cases, the bypass must replace all the circulation to a major arterial territory, and therefore a large conduit is needed. The normal blood flow of the MCA is about 250 mL/min to the cerebral hemisphere. The blood flow of the PCA is only moderately less. The average STA graft provides blood flow of just 15 to 30 mL/min, although it may increase with time.50–52 Blood flow through a saphenous vein graft typically ranges from 70 to 140 mL/min and can exceed 250 mL/min.51,53 Blood flow through a saphenous vein graft, which averages about 4 to 5 mm in diameter, is high enough to support the circulation in an entire major arterial territory at a level well above the ischemic threshold (particularly when added to the variable contribution through leptomeningeal collateral vessels). Despite these advantages, a vein graft generally has lower long-term patency rates and a higher risk of kinking, and there can be problems with caliber mismatch between the larger vein and smaller intracranial vessels. Alternatively, a radial artery graft can be used, which has a smaller diameter (about 3.5 mm) and a flow rate between 40 and 70 mL/min.51,54 A type II bypass can be a substitute for an STA-MCA bypass when the scalp artery is hypoplastic, diseased, or occluded. In a patient with an aneurysm that can be occluded only proximally, as in the case of some dolichoectatic and fusiform aneurysms, our experience has been that a type II bypass may supply too much flow and can be dangerous. With excessive retrograde filling from a robust saphenous vein graft, the aneurysm can remain patent, continue to enlarge, or even rupture in some cases. In these circumstances, if the distal aspect of the aneurysm must be left unoccluded, we prefer the use of an STA type III bypass when possible.
Extracranial Carotid Artery–to–Middle Cerebral Artery Saphenous Vein Interposition Graft
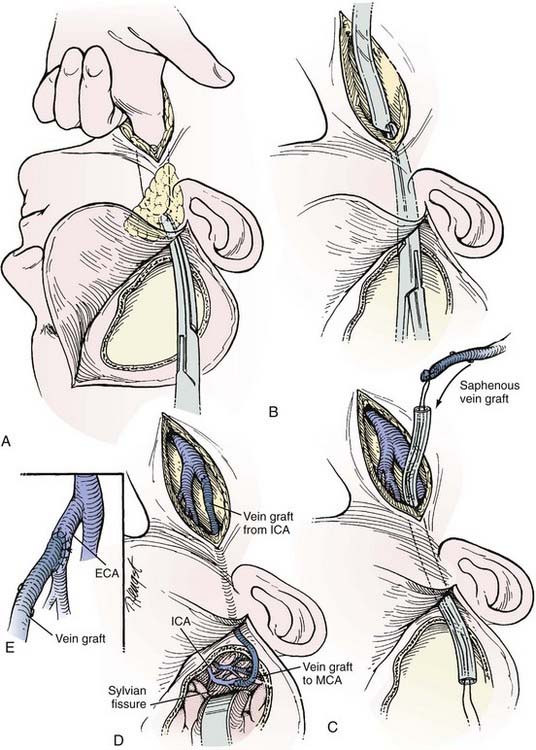
The saphenous vein graft is typically connected end to end to the proximal stump of the ICA or end to side to the ECA (see Figs. 380-4 and 380-5). For the distal anastomosis, we prefer an end-to-side anastomosis to a larger, more proximal MCA branch (M2 or M3 segment) in the sylvian fissure. These vessels better match the size of the saphenous vein and provide a more direct conduit to the entire MCA territory.
After the carotid bifurcation is exposed and a pterional craniotomy performed, the sylvian fissure is opened widely. The ideal M2 or M3 arterial recipient site, free of perforating vessels, is exposed. Next, the saphenous vein is exposed and isolated but left in situ in continuity until just before it is used for the bypass. At our institution, we have a highly experienced cardiac surgery fellow or cardiac surgery physician’s assistant harvest the vein graft by endoscopic technique. Meticulous care is exerted while exposing the vein to avoid trauma that might cause the bypass to thrombose.55–57 The alignment of the vein should be marked with a 6-0 Prolene suture (Ethicon, Johnson & Johnson Professionals, Inc., Somerville, NJ) through the adventitia to define the proper orientation of the vein. This maneuver avoids twisting the vein as it is positioned for the bypass. The vein is ligated proximally and distally, excised, and flushed without overdistention with cool, heparinized saline.
As suggested by Sundt and associates,4 the intracranial anastomosis is performed first. This sequence allows the surgeon to take advantage of slack in the graft, which can be manipulated freely while the back and front walls of the anastomosis are sutured. The terminal 5- to 6-mm portion of the vein graft is trimmed of loose adventitia, and the end is beveled to create an orifice 5 to 6 mm in diameter. After barbiturates are administered and blood pressure is stabilized at 10% to 20% above the patient’s baseline, a 10- to 15-mm length of MCA is occluded between temporary clips. A linear MCA arteriotomy is matched to the diameter of the vein graft orifice. The vein graft is fixed to the MCA branch with 8-0 monofilament nylon sutures, which are used to complete a running closure. After the anastomosis is completed, blood flow is restored, and the barbiturates can be stopped.
As an alternative procedure, a short vein graft can be placed between the STA trunk (exposed at the zygomatic arch) and a proximal M2 or M3 branch of the MCA.58 This procedure is useful when only a short segment of saphenous vein is available or the cervical carotid artery cannot be used as the site of the proximal anastomosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree