CHAPTER 390 Carotid-Cavernous Fistulas
The term carotid-cavernous fistula (CCF) refers to an abnormal communication between the internal carotid artery (ICA) or one of its branches or the external carotid artery (ECA) and the cavernous sinus (CS). Over the years, numerous classifications have been applied to CCFs.1 They are based on angiographic features (high-flow and low-flow fistulas), mechanism of onset (spontaneous and traumatic), morphology, and angioarchitecture (direct and indirect fistulas). Based on arterial supply, Barrow and colleagues classically divided CCFs into four types.2 A type A fistula is a direct communications between the ICA and the CS; type B fistulas are supplied exclusively by dural branches of the ICA and are uncommon. Supply to type C fistulas is provided by dural branches of both the ICA and the ECA, whereas type D fistulas are supplied exclusively by dural branches of the ECA. The Barrow classification has been widely adopted. However, from a practical, etiopathogenetic, clinical, and therapeutic point of view, the simplest and most useful classification divides CCFs into direct and indirect. These two types of fistulas are considered separately in this chapter.
Anatomy
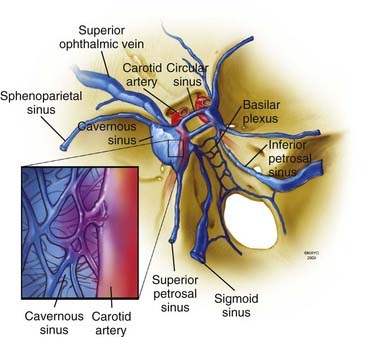
A brief overview of the general, arterial, and venous anatomy of the CS is important for the understanding of CCFs and their treatment. The CS or, more properly, the lateral sellar compartment is an anatomic extradural space in direct continuity through the clivus and basiocciput with the epidural space surrounding the spine.3 The CS is an extradural space contained between the two layers of dura laterally and superiorly and the periosteum covering the lateral portion of the sphenoid sinus and the sphenoid bone inferiorly and medially. This extradural space contains the ICA with its typical S shape, as well as nerves, fat, and a plexus of veins.3 Therefore, the CS is not, as classically described in older anatomic textbooks, a single venous space through which the ICA and the cranial nerves course. The plexiform arrangement of the veins in the CS was apparent to Dwight Parkinson when he entered this space to directly obliterate a long-standing CCF.3 He remarked that “the engorged and thickened ‘arterialized’ veins were readily noted to be neither cavernous nor a dural sinus but a plexus of veins.”3 This observation explains “the compartmentalization” of the CS often noted during endovascular approaches to CCFs.
The ICA gives origin to a variable number of small arterial branches during its course in the CS. The most consistent one is the meningohypophysial trunk arising before the apex of the first curve of the ICA. This vessel provides arterial supply to the dura of the tentorium (tentorial artery or artery of Bernasconi-Cassinari) and the hypophysis (inferior hypophysial artery). Other fairly constant branches are the inferolateral trunk, which supplies the segment of the cranial nerves running into the lateral sellar compartment, and the capsular artery of McDonnel. There are constant anastomoses between branches of the ICA and the ECA. Although not always angiographically visible, these anastomoses are anatomically constant. Knowledge of these anastomotic channels is very important to prevent complications during endovascular approaches to indirect CCFs.4
Understanding of the venous channels connected to CS veins is key to interpretation of the angiographic anatomy of CCFs and to plan their treatment (Fig. 390-1). The anterior portion of the CS receives the superior and inferior ophthalmic veins and the sphenoparietal sinus. The two CSs communicate through the anterior and posterior intercavernous sinus forming the circular sinus. Posterior drainage is through the basilar plexus and the superior and inferior petrosal sinuses. Inferolaterally, connections exist through dural veins draining into the pterygoid plexus. Intermittently, the cavernous sinus can receive drainage from the inferomedial surface of the brain.
Clinical Findings
The clinical findings and initial symptoms and signs are primarily related to the pattern of drainage of the fistula and its rapidity of development. As a general rule, direct fistulas have a more dramatic manifestation and quite often tend to exhibit the “classic” triad of exophthalmos, chemosis, and visual loss. In a large series of patients with direct CCFs, the most common signs and symptoms at initial evaluation were an orbital bruit (80%), proptosis (72%), chemosis (55%), cranial nerve VI palsy (49%), complete ophthalmoplegia (24%), and visual loss (18%).5 Indirect fistulas often have a more subtle clinical course, and not unusually patients have few symptoms, often transient. In the setting of major facial and head trauma, a CCF can easily be overlooked, so it is good practice to auscultate over the eyelid to rule it out. Diplopia is common and related to ischemic dysfunction of cranial nerves, mechanical compression of the nerves, and restricted movement secondary to venous engorgement of the orbital contents. In patients with direct fistulas, cranial nerve VI is more commonly involved because of the contiguity of the carotid artery to this nerve. The pattern of double vision created by engorgement of the orbital contents and restricted movement may not fit with a particular cranial neuropathy. Venous drainage is also dependent on the anatomic location of the fistula. Anteriorly placed fistulas drain through the superior ophthalmic vein and have a significant exophthalmic component.6 Posterior fistulas often have contralateral involvement. Epistaxis, even fatal epistaxis, is not uncommon with a traumatic direct CCF and can occur in delayed fashion, weeks after the original trauma.7 Intracranial hemorrhage can also occur with both direct and indirect fistulas associated with retrograde cortical venous drainage. Intracranial hemorrhage in the setting of a direct CCF frequently portends a poor prognosis with a high risk for short-term rehemorrhage. Thus, treatment should be considered on an urgent basis in such a situation.
Imaging Studies
Axial imaging studies such as computed tomography and magnetic resonance imaging can show indirect signs such as fullness in the area of the CS, proptosis, or pathologically enlarged venous channels. However, despite the sophistication of modern imaging techniques, catheter-based angiography remains the “gold standard” for diagnosing and defining CCFs. A cerebral angiogram must include the internal and external carotid supply, as well as study of the contralateral side and posterior circulation. Selective injection of the external carotid system is important to define the supply and angioarchitecture of indirect CCFs. With direct fistulas, it can be difficult to exactly define the location and size of the fistulous tract because of high flow. Vertebral angiography with compression of the carotid artery in the neck usually localizes the fistula by demonstrating slow filling though the posterior communicating artery (Huber’s maneuver).8 Similarly, selective injection of the ICA while gently compressing the common carotid artery may help localize the fistula (Mehringer-Hieshima maneuver). On rare occasion, the ICA proximal to the fistula may have been dissected by the injury, and this situation may compromise the ability to safely catheterize the vessel.
Direct Fistulas
Direct CCFs can be divided into traumatic and spontaneous. In large series, 80% to 90% of direct CCFs are traumatic,6 the result of direct or indirect trauma involving the creation of a pathologic communication between the cavernous ICA or less commonly one of its branches and CS veins. The propensity of this segment of the ICA to rupture is probably related to tethering of the intracavernous ICA by dural rings at its entrance into and its exit from the CS. Iatrogenic fistulas have been reported after various surgical procedures, including transsphenoidal resection of pituitary tumors, attempts at reopening an occluded ICA with a Fogarty balloon, and endoscopic procedures. Much less common are spontaneous CCFs occurring as a result of rupture of an intracavernous aneurysm (Fig. 390-2) or as part of congenital connective tissue defects, such as Ehlers-Danlos type IV syndrome.
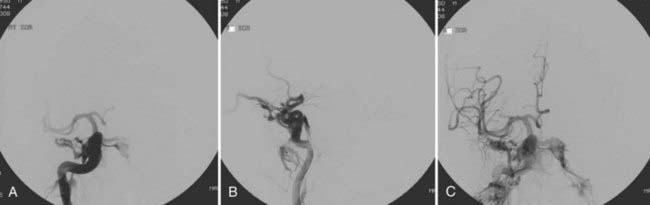
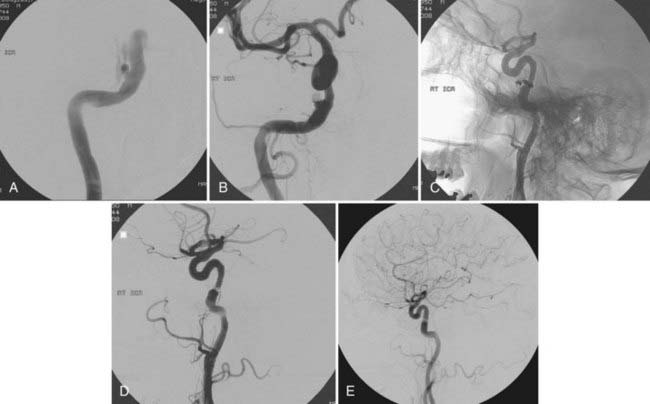
FIGURE 390-2 Selective right internal carotid artery injection. Anteroposterior (AP) (A) and lateral (B) early arterial phase images show a direct carotid-cavernous fistula with anterior and posterior drainage. An AP late arterial phase image (C) shows opacification of the contralateral cavernous sinus through the intercavernous sinus. This 60-year-old man had an abrupt onset of disabling bruit secondary to spontaneous rupture of a small intracavernous internal carotid artery aneurysm (see Fig. 390-3).
Treatment of Direct Carotid-Cavernous Fistulas
Detachable balloons were introduced for the treatment of CCFs by Serbinenko in 1974 and have provided a valid solution to the majority of direct CCFs for the past 3 decades.9 Balloons can be flow directed through the orifices of the fistula, inflated in the venous side up to a size larger than the original communication to prevent herniation into the ICA, and then detached. With a detachable balloon, closure of the fistula with preservation of the ICA is possible in approximately 75% to 80% of cases.5,6 Reconstruction of the parent vessel with this technique is not always perfect, and an irregularity at the level of the ICA tear with protrusion into the CS can be seen in up to 50% of patients after successful treatment with detachable balloons.6 Long-term follow-up of these wall irregularities is important because enlargement has been observed in up to 30% of patients.10 Limitations of detachable balloons for the treatment of CCFs include premature detachment with the danger of distal embolism, delayed balloon deflation with recurrence of the fistula, and rupture of the balloon because of osseous spicula in the presence of traumatic fractures with subsequent deflation and recurrence of the fistula. In some cases it can be difficult to navigate the balloon through a small communication; similarly, in the presence of a large communication between the artery and the venous side, balloons may be inadequate to close the fistulous tract and the ICA may need to be sacrificed. After detachable balloon treatment, up to a third of patients can experience worsening of preexisting or new extraocular motility deficits. These deficits often involve the sixth cranial nerve running between the ICA and the lateral wall of the CS. Postoperative deficits are usually transient but can be permanent in about 15% of patients.6 Ischemic complications have been reported in 7% of patients (3% transient and 4% permanent).5 Since 2003, detachable balloons have not been available in the United States.
With the availability of detachable coils beginning in the early 1990s, these devices have commonly been used for transarterial or transvenous closure of direct CCFs. Detachable coils overcome some of the problems encountered with detachable balloons. Moreover, with withdrawal of detachable balloons from the U.S. market, detachable coils have become the mainstay of treatment of direct CCFs in the United States (Fig. 390-3). The main advantage of detachable coils is their ability to be retrieved in the event of inadequate placement. Coils are available in different sizes and shapes and can be easily adapted to the individual characteristics of the specific patient. From a historical point of view, the first clinical case in which Guglielmi Detachable Coils (GDCs, Boston Scientific, Natick, MA) were used involved a patient with a CCF.11 HydroCoil (MicroVention, Inc., Tustin, CA), which is a coil coated with a gel that “swells” when in contact with blood, has recently also been used successfully for the obliteration of direct CCFs.12 Because of the properties of these coils and the ability of the coil mass to “expand” after deployment, a smaller number of coils are required to achieve occlusion. When deploying coils, if retrograde cortical venous drainage exists, it is important to obliterate this portion of the fistula first with coils to avoid redirecting residual flow preferentially to this portion of the fistulous communication and incur the risk of intracranial venous engorgement and hemorrhage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree