Safety and Complications
Key Points
A methodical team-based and system-based approach to safety is becoming more established in the culture surrounding medical procedures.
The recent reduction in the volume of diagnostic cerebral angiography due to the advent of noninvasive imaging compels trainees to learn basic skills only on complex interventional cases.
Angiographic complications share much in common with dangers in other fields touched uncommonly but dramatically by disastrous calamities. Road accidents, aviation crashes, and so on, do not happen spontaneously. They are caused when a concatenation of circumstances align at a particular point in time, and the preventive safety measures in place to avoid these accidents have either been neglected or failed to take account of the particular factors in play. Generally, however, the influential circumstances are banal rather than exotic. A driver reaches for a pen to jot down a note when speaking on his/her cell phone. Pressed for time to return home, a single-engine pilot decides to race ahead of the projected weather front moving in. Most “accidents” evolve from the mundane. Similarly, during angiographic procedures, while exotic possibilities do exist, such as a spontaneous aortic perforation by the wire in an Ehlers–Danlos patient or an anaphylactic reaction, they are extremely rare. Most complications are triggered by drearily commonplace circumstances. A tired technologist reaches for an exchange-length microwire instead of a standard-length wire. An inexperienced angiographer decides that he/she is quite capable of performing an epistaxis embolization. Thwarted by the refusal of a wire to enter a particular branch vessel, an angiographer decides that he/she is going to teach that wire a lesson it will never forget and make it go.
Prompted by a growing public awareness of the potential magnitude of the prevalence of medical errors (1), the Joint Commission (2003) (2) and subsequently the World Health Organization (WHO) (3) have released recommendations for Surgical Safety Checklists and Time-Outs, which have been implemented in operating rooms and are becoming standard in endovascular suites. Discussions of the importance of teamwork and the need to communicate clearly, due to the much hackneyed nature of these phrases, can tend to draw a response of yawning lip-service. Nevertheless, it is important to be aware that an extensive medical literature upholds the validity of intervention aimed at changing personal attitudes and institutional culture (4). Evidence demonstrates a reduction in wrong site/wrong procedure surgeries, improved early reporting of equipment problems, avoidance of medication problems, and reduced operational costs (5). Some of these lessons are of limited value, obviously, to a field in which direct visualization of the lesion would preempt the possibility of wrong-side surgery. However, data concerning staff attitudes to and perception of the success of teamwork show a discrepancy between those of nurses, for instance, and physicians. In one study with a ring of verisimilitude to it, nurses described good collaboration as “having their input respected,” whereas physicians often described good collaboration as having nurses “who anticipate their needs and follow instructions” (4). It would not be difficult, therefore, to understand how some measure of reluctance to voice qualms during a procedure on the part of technologist or nursing staff might easily establish itself to the detriment of patient safety.
Comparisons with the culture of professional aviation confirm that a substantial lag exists in making progress within the medical profession of the type that has been wrought over 50 years of Crew Resource Management Training in the flying world. A culture of hiding errors or ignoring them as a “one-time” event still persists. In one study comparing physicians and pilots, 60% of physicians (70% of surgeons in the study) stated that even when fatigued they were capable of working with complete effectiveness at critical times, whereas the corresponding response from officer pilots was a modest 26% (6). Taking the lead from the aviation industry, the medical world in now making progress with a greater emphasis on medical team training rather than individual training and on postprocedure debriefings and analysis with open sharing of information (7).
Just as the surgical world has been lagging behind the safety standards of commercial aviation, it is safe to say that the neuroendovascular field is still further behind. At this time, no clear consensus or professional guidelines exist, for instance, on the necessity or value of prophylactic administration of antibiotics during percutaneous procedures, or use of antiembolic devices during prolonged procedures. These decisions are still made anachronistically on an institutional or individual physician basis. Furthermore, there are aspects of the field that do not lend themselves well to institutional regulatory oversight. Standards to establish or prove competence in the field in general or with particular devices are virtually nonexistent or perfunctory. While the point has been made that comparing the aviation industry track record on safety with the medical world can only go so far before the analogy breaks down (8), not least because in the aviation
world when there is an accident the pilot will be the first one hurt, hence the motivation factor, nevertheless, considerable room for improvement exists in our field (Figs. 5-1 and 5-2).
world when there is an accident the pilot will be the first one hurt, hence the motivation factor, nevertheless, considerable room for improvement exists in our field (Figs. 5-1 and 5-2).
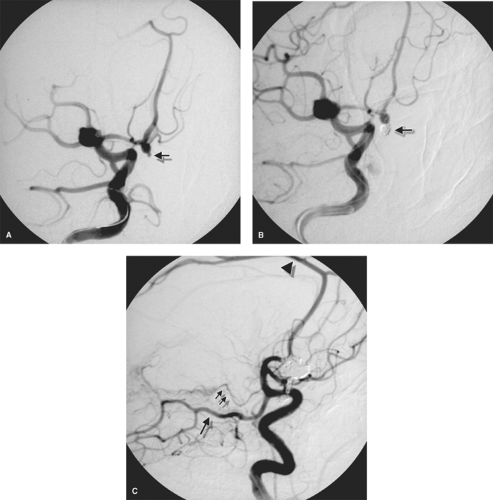
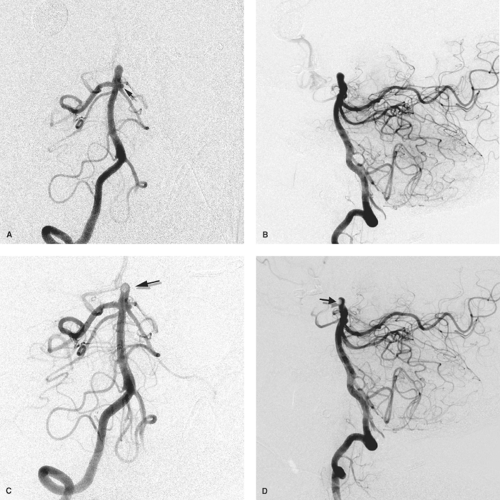
 Figure 5-1. (A–C) Why fellowship training is necessary. This young female presented with recurrent epistaxis, but was well-controlled with first aid measures and could have been transferred to a tertiary care center. A physician at her community hospital undertook to perform particle embolization of the external carotid artery bilaterally. The images of the right external carotid artery anteroposterior (AP) pre-embolization (A), lateral pre-embolization (B), and lateral postembolization (C) are presented. The patient’s left-sided images are presented in Chapter 25. The images demonstrate a rare but crucial-to-recognize variant of the ophthalmic artery (arrow) arising from the middle meningeal artery. The distal internal maxillary artery (double arrowheads) demonstrates a fairly standard configuration, as it breaks up into the long and short sphenopalatine branches in the pterygo-maxillary fissure. Unfortunately for the patient, the physician embolized the internal maxillary artery in a way that occluded both the ophthalmic artery and the internal maxillary artery. The result was that the patient experienced excruciating pain in the right eye and immediate loss of vision, which prompted cessation of the case moments before embolization of the left external carotid artery was to begin. |
Neurologic Complications of Cerebral Angiography
Since the first edition of this text, the population of patients undergoing neuroendovascular procedures has changed considerably. Diagnostic angiographic procedures have been supplanted in large measure by noninvasive imaging. On the other hand, therapeutic procedures have become more complex and reaching in their scope. One result of this change is that the older literature pertinent to procedural complications based on patients undergoing diagnostic procedures for evaluation of carotid artery disease, subarachnoid hemorrhage, or questionably ischemic symptoms has become outdated. While some of these patients are still undergoing endovascular procedures—those with atherosclerotic disease are being catheterized with 6F to 8F introducer devices instead of what would have been previously a 5F diagnostic catheter, and subarachnoid hemorrhage patients are being treated definitively with invasive procedures involving balloon catheters, stents, and liquid or coil devices—conclusions from the older body of literature cannot be extrapolated reliably to the modern
practice of neuroangiography. With a few exceptions, a modern body of literature dealing with this topic has not emerged as yet. Many of the older papers referenced below, however, are still highly relevant to particular groups of patients, such as those with carotid artery disease in the setting of angioplasty and stenting procedures.
practice of neuroangiography. With a few exceptions, a modern body of literature dealing with this topic has not emerged as yet. Many of the older papers referenced below, however, are still highly relevant to particular groups of patients, such as those with carotid artery disease in the setting of angioplasty and stenting procedures.
The safest endovascular procedure is the one that can be cancelled as unnecessary or that can be avoided by performing noninvasive imaging (Fig. 5-3). Complications include the possibility of killing the patient, and this risk exists with every patient who entrusts his/her welfare to your hands. The need for excellent technical training backed up by a thorough knowledge of the diseases of the neurovascular system is self-evident. Many serious complications of angiography are made worse by the effects of continuing the study, oblivious to a developing problem (Table 5-1;Figs. 5-3–5-5).
Clinically Detectable Neurologic Complications
Older studies of complications of cerebral angiography, based on clinically evident neurologic complications arising within 24 to 72 hours of transfemoral cerebral angiography, report an overall incidence of approximately 0% to 6.8% (9,10,11,12,13,14,15,16,17,18,19,20,21,22). The majority of these events are undoubtedly transient, but there is a deal of latitude in the literature as to how many permanent neurologic complications can be seen. Figures as high as 5.7% are sometimes quoted for enduring neurologic deficits, but a more realistic figure for reasonably experienced angiographers might be in the region of 1.2%, as reported in the asymptomatic carotid artery stenosis (ACAS) study (17). Studies of pediatric patients in specialized tertiary referral hospitals report a 0% complication rate in retrospective studies (23,24).
Some studies contain a bias in that they consider neurologic events to be angiographic complications only if they appear to be related to the angiogram in the opinion of the investigator. Some studies (12,25,26) have managed to avoid this bias by including all neurologic deteriorations occurring within 24 hours of a cerebral arteriogram. They have found neurologic deterioration rates of approximately 9% to 14.7% for all neurologic events (18) and 0.4% to 5% for permanent events in patients referred for evaluation of recent ischemic disease. Many of the recorded complications were exacerbations of previous conditions. Earnest et al. (12) speculated that compromise of cerebral blood flow in this group of patients might be a final common pathway for many of these complications. Likely causes include inadequate hydration or normalization of blood pressure before the angiogram in patients for whom cerebral circulation is dependent on a hypertensive baseline state. On the other hand, consideration must be given to the rate of spontaneous disease-related, rather than procedure-related, events in this population over a similar period. Baum et al. (27) studied a group of patients whose angiographic examinations were canceled, and who demonstrated a similar incidence of clinical events in the time period that would have been considered in evaluation of postangiographic complications.
Variable features among studies of complications after cerebral angiography include the patient population and diseases, referral characteristics of the institution, whether the study took place at a teaching hospital, the changing practice of radiology depending on the year of the study, and whether the postangiographic evaluation was done by a radiologist or a neurologist. Risk factors for clinically evident neurologic complications in the first 24 hours after cerebral angiography include:
Patients whose angiograms demonstrate greater than 50% to 70% stenotic disease of the cerebral vessels (14,16,22,25).
Patients whose angiogram was performed by a fellow alone (22).
The risk of cerebral angiography performed by trainees at teaching hospitals has been examined by a number of studies (9,11,13,27). Some studies (10,22,29) concluded that difficulty in catheter manipulation by inexperienced radiologists was a risk factor for the examination taking more time, possibly in turn creating a higher likelihood of neurologic complications. Moreover, there is a definite historical trend toward trainees having a lower volume of diagnostic neuroangiographic studies under supervision. Noninvasive imaging has had an impact on the volume of cases, and programs have expanded the numbers of trainees, thus changing the denominator for the available volume of cases. Gabrielsen (30) has commented that complication rates at many institutions are probably substantially higher than those quoted in medical journals, pointing out that a diminution of the workload in cerebral angiography implies that trainees and practicing neuroradiologists now gain less expertise than 20 years ago. In response to this, the American Society of Neuroradiology and allied societies in the neurovascular field issued a joint statement in 2004 suggesting that a case log of 100 patients become the minimum standard for establishing adequate training in diagnostic cervicocerebral angiography (31,32).
Table 5-1 General Principles for Avoiding Complications of Cerebral Angiography | |||
|---|---|---|---|
|
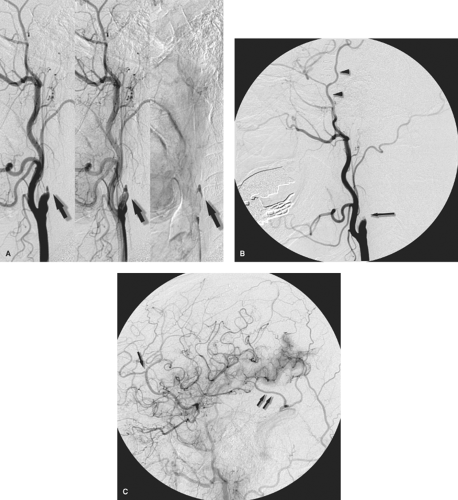 Figure 5-7. (A–C) Stump emboli. The phenomenon of stump embolization can come up after several surgical or endovascular procedures in which a segment of occluded vessel becomes the source of fresh thrombus entering the flowing artery to the brain. Any type of arterial sacrifice for tumor control, trapping of a pseudoaneurysm, or treatment of a carotid cavernous fistula can set up the circumstances where this rare but serious complication can be seen. In this patient presenting with acute occlusion of the left internal carotid artery, a left common carotid angiogram (A) shows the left internal carotid artery stump with complete occlusion. In retrospect, the progressive images of the run showed a dynamic evolving quality of the stagnant contrast (arrows in A) within the occluded artery. These findings would not be evident in a long-occluded vessel. The patient underwent a left superficial temporal artery to middle cerebral artery bypass for relief of fluctuating ischemic symptoms, but developed severe postoperative complications of infarctions of the left hemisphere. A postoperative angiogram (B, lateral view) shows that the bypass (arrowheads in B) is widely patent, but the configuration of the internal carotid artery stump appears different now (long arrow in B). The left common carotid artery injection centered over the head (C, lateral view) shows excessive parenchymal blush in the region of the left temporal lobe with early venous opacification of the vein of Labbé (double arrow in C) indicating the effects of cerebral infarction. Several filling defects were suspected within the arteries on other images, one of which is seen in the pericallosal artery (single arrow in C). The source of the emboli was thought to be reflux of fresh thrombus from the still evolving response to occlusion of the left internal carotid artery stump.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|