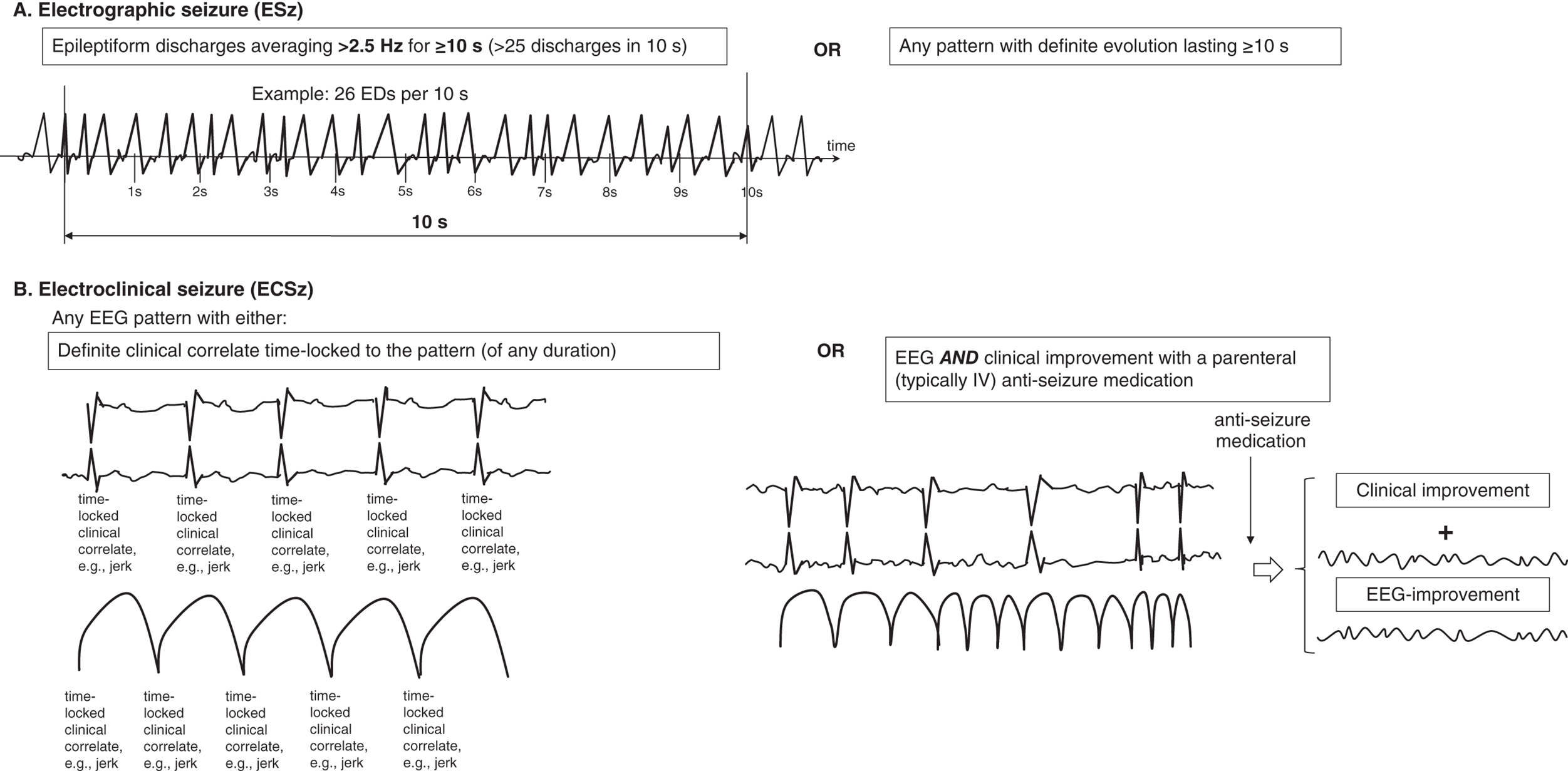
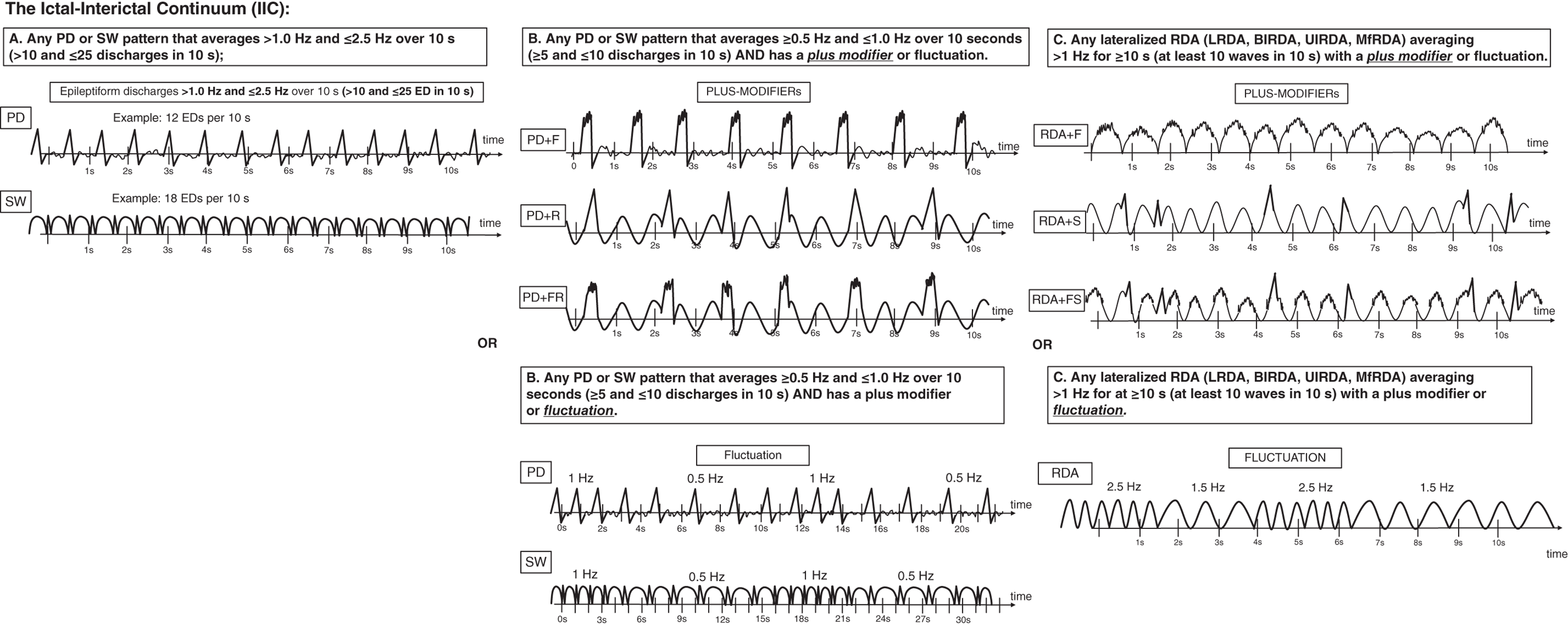
Seizures are very common in the ICU, particularly in patients with acute or chronic brain injuries. The prevalence of seizures in those undergoing continuous EEG monitoring (generally for 24 hours or more) ranges from 8% in those without prior seizures and with no subtle signs of seizures to 48% after convulsive status epilepticus. Most studies of acute brain injury (including traumatic brain injury, intraparenchymal hemorrhage and subarachnoid hemorrhage) show a prevalence of seizures of 15–40%. It is clear from many studies now that the majority of seizures in the critically ill are nonconvulsive and can therefore only be diagnosed via EEG. Seizures appear to be an independent predictor of worse outcome in multiple populations, including worse long-term functional outcomes, cognition, hippocampal atrophy and later epilepsy. Electrographic seizures (ESz) are defined by the ACNS critical care EEG terminology 2021, as either (Figure 6.1): Electroclinical seizures (ECSz) are defined as any EEG pattern with either (Figure 6.1): Seizures in the critically ill with encephalopathy tend to be of slower frequencies, last longer and have less clearly defined onset, evolution and offset than seizures in awake patients. Thus, they can be more difficult to recognize, both visually and via computer-based detection. In general, there must be clear evolution in frequency, morphology or location of an ongoing EEG pattern to be sure it represents seizure activity. However, in prolonged nonconvulsive seizures, the evolution can be subtle or even absent. Status epilepticus in concept basically refers to ongoing and prolonged seizure activity. A strong misconception among clinicians is that all status epilepticus is the same. It must be stressed that just as seizures can be highly variable (e.g., absence vs. focal seizure with impaired awareness vs. generalized tonic-clonic), the states and patterns that meet criteria for status epilepticus are also highly variable. These varied presentations have significantly different associations with neuronal injury and therefore can vary greatly with regard to how aggressively/urgently they need to be treated. This diversity was reflected in the ILAE position statement on the definitions and classification of status epilepticus. Electrographic status epilepticus (ESE) is defined by the ACNS as an ESz for ≥10 continuous minutes, or for a total duration of ≥20% of any 60-minute period of recording. Previously, it was common to require ESzs to occupy ≥30 minutes (or ≥50%) total duration of any 60-minute period of recording to qualify as ESE. The reduction to ≥20% is largely based on one large study that demonstrated that a seizure burden of >20% was associated with significantly increased chance of neurological decline in a cohort of critically ill children. A similar cutoff was identified in neonates with hypoxic ischemic encephalopathy. Multiple other studies in a variety of ages and conditions have shown a ‘dose-response’ effect, with greater seizure burden (either measured as peak burden in 1 hour or in 12 hours, or cumulative number of hours) resulting in worse outcomes, both short-term and long-term. Electroclinical status epilepticus (ECSE) is defined as an ECSz for ≥10 continuous minutes, or for a total duration of ≥20% of any 60-minute period of recording. An ongoing seizure with prominent bilateral motor activity (and impaired awareness) only needs to be present for ≥5 continuous minutes to qualify as ECSE. This can also be referred to as ‘convulsive SE’, a subset of ‘SE with prominent motor activity’. In any other clinical situation, the minimum duration to qualify as SE is 10 minutes. Some of the more common types of status epilepticus include: In the ICU there are many patients where it is uncertain if the ongoing electrographic pattern is contributing to their clinical signs. These patients are classified in the category of ‘possible ECSE’. It should be noted that if it is certain that the electrographic pattern is causing clinical signs (irrespective of the pattern), this would qualify as ECSE. ‘Possible ECSE’ is defined as a RPP that qualifies for the ictal-interictal continuum (IIC) (i.e., it does not qualify as a ESz/ESE: further discussed and defined below) that is present for ≥10 continuous minutes, or for a total duration of ≥20% of any 60-minute period of recording, which shows EEG improvement with a parenteral anti-seizure medication BUT without clinical improvement. This remains largely in line with ‘possible NCSE’ as defined by the Salzburg criteria, commonly accepted criteria for electrographic SE that were largely incorporated into the ACNS terminology. A specific population that can pose diagnostic challenge are patients with a known prior epileptic encephalopathy. In these patients the baseline EEG is often severely abnormal (at times meeting criteria for ESE). For these patients to qualify as having ECSE, the EEG pattern needs to represent either: The definitions of what constitutes seizures are mostly based on historical consensus. For example, there is little evidence that a 2.3-Hz pattern causes less neuronal injury than a 2.7-Hz pattern. Yet one is classified as an ESz (or ESE if persistent) and the other is not. There are many patterns (that do not qualify as ESz/ESE) that appear clinically worrisome, can cause subtle clinical signs, are associated with ictal physiology (e.g., increased blood flow and metabolism), and could contribute to additional neuronal injury, especially in the setting of acute brain injury. Thus, they are potentially ictal in some sense. These patterns fall on the ‘ictal-interictal continuum’, a synonym for ‘possible status epilepticus’. When patients with chronic epilepsy are monitored in the epilepsy monitoring unit (EMU) there is a clear distinction between ictal and interictal states (i.e., what is seizure, and what is not). There is also, for the most part, a crisp and immediate transition between these two states, with a clearly identifiable seizure onset, and offset. In critically ill patients the mechanisms of seizure generation, and perhaps more importantly the maintenance of seizure cessation, is often severely impaired. In these patients there is commonly a gradual waxing and waning between patterns, ranging from those that are fairly benign and those that are more epileptic, and at times ESz/ESE. This phenomenon resulted in the term ictal-interictal continuum (IIC). The definition of the IIC is broad and deliberately inclusive, mostly to stimulate further studies into differentiating between patterns that are potentially injurious vs. those that are not. Patterns currently thought to qualify as the IIC, based on the ACNS definition in 2021, include (Figure 6.2): and For the most part, the primary goal of cEEG is to detect seizures as quickly as possible, and have this information fed back to clinicians to allow prompt treatment in order to improve outcomes. Although many centers have a dedicated service in order to do this, there are often delays in setting up the EEG, which requires technical training, and reviewer feedback to clinicians. To avoid these delays, there have been systems/devices (caps, belts, headbands, etc.) developed that allow for the rapid application (within a few minutes) of EEG with minimal training that allows instant review by clinicians and/or has automated interpretation. There is even the feature to ‘sonify’ the EEG (i.e., convert the EEG waves into sound waves) so clinicians can learn to distinguish the constant hum of a non-epileptiform record, versus a louder, more irregular and sometimes crescendo pattern of seizures or status epilepticus, which requires little to no knowledge of the EEG to interpret. Through these means detection of seizures and feedback to clinicians can often occur markedly faster (minutes rather than hours or longer) compared to conventional cEEG. The limited montages of several of these devices do have a mildly reduced sensitivity and specificity compared to traditional full montage; however, rapid EEG is becoming an important supplement to conventional cEEG in many centers for the benefits described, and an important new option for centers without capability for rapid EEGs (especially off-hours) or continuous EEG monitoring. EEGs throughout this atlas have been shown with the following standard recording filters unless otherwise specified: LFF 1 Hz, HFF 70 Hz, notch filter off. *Reference defining the terms used in this atlas. Figure 6.1. Electrographic and electroclinical seizures. Electrographic seizures (ESz) are defined as either: 1. epileptiform discharges averaging >2.5 Hz for ≥10 s (>25 discharges in 10 s) (panel A), or 2. any pattern with definite evolution and lasting ≥10 s. Electrographic status epilepticus (ESE) is defined as an electrographic seizure for >10 continuous minutes or for a total duration of >20% of any 60-minute period of recording. Electroclinical seizure (ECSz) is defined as any EEG pattern with either: 1. definite clinical correlate time-locked to the pattern (of any duration, including subtle clinical correlate as long as it is time-locked to the pattern) (panel B), or 2. EEG and clinical improvement with a parenteral (typically IV) anti-seizure medication. The EEG pattern during an ‘electroclinical seizure’ does not necessarily need to qualify as an ‘electrographic seizure’. For example, if static 1-Hz PDs have a clinical correlate, this would not qualify as an electrographic seizure, but would qualify as an electroclinical seizure (as shown in panel B). Many seizures would however qualify for both ‘electrographic’ and ‘electroclinical’ seizures, and these should be reported under both terms. Electroclinical status epilepticus (ECSE) is defined as an electroclinical seizure for >10 continuous minutes or for a total duration of >20% of any 60-minute period of recording. An ongoing seizure with bilateral tonic-clonic (BTC) motor activity only needs to be present for >5 continuous minutes to qualify as ECSE. This is also referred to as ‘convulsive SE’, a subset of ‘SE with prominent motor activity’. In any other clinical situation, the minimum duration to qualify as SE is >10 minutes. ‘Possible ECSE’ is an RPP that qualifies for the IIC that is present for ≥10 continuous minutes or for a total duration of >20% of any 60-minute period of recording, which shows EEG improvement with a parenteral anti-seizure medication BUT without clinical improvement. This remains largely in line with ‘possible NCSE’ as defined by the Salzburg criteria. Reproduced from Hirsch LJ, Fong MWK, Leitinger M, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. J Clin Neurophysiol. 2021;38(1):1–29, with permission. Figure 6.2. Ictal-interictal continuum (IIC).
6
Seizures, status epilepticus and the ictal-interictal continuum
6.1 Electrographic and Electroclinical seizures
6.2 Status epilepticus
6.3 Ictal-interictal continuum (IIC)
6.4 Rapid EEG
Figure list
Suggested reading
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


