Chapter 11 Sella and Central Skull Base
ANATOMY
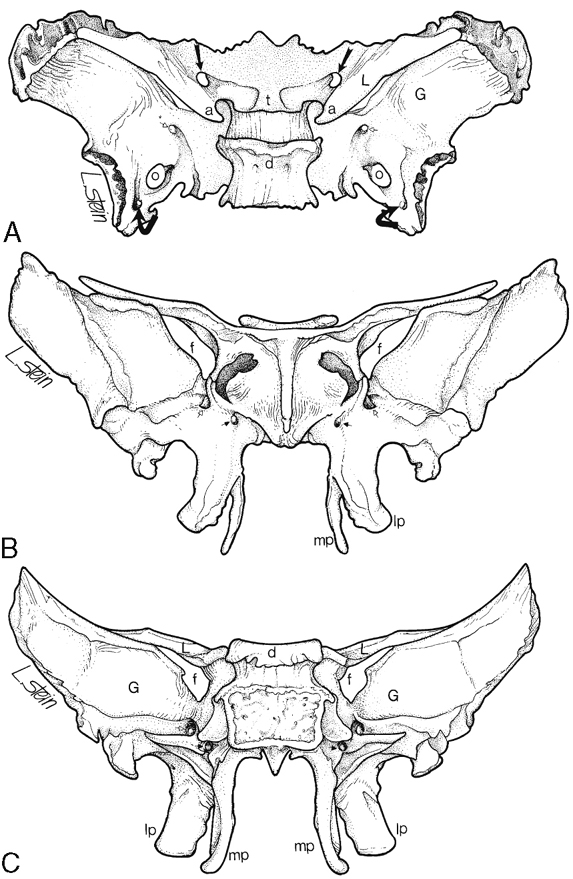
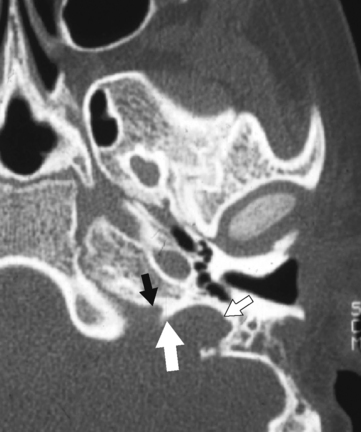
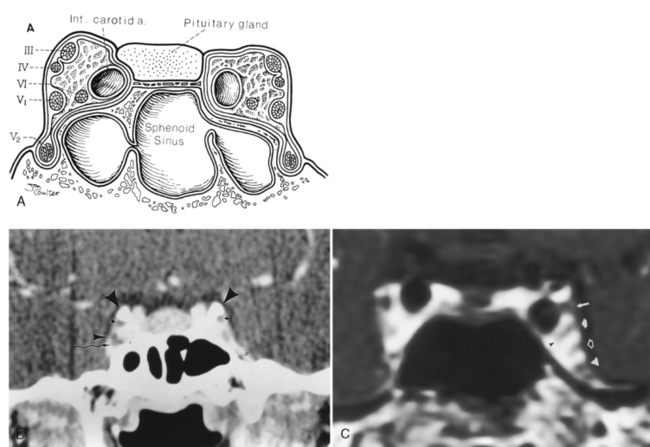
Appreciation of the normal anatomy is the key to the radiologic evaluation of the sella and central skull base. Small lesions can have profound endocrinologic and neurologic manifestations. We define the skull base as the region from the upper surface of the ethmoid bone and orbital plate of the frontal bone to the occipital bone. Central to the skull base is the sphenoid bone. The bone itself has the appearance of a bat with its wings extended (Fig. 11-1). The feet of the bat are the medial and lateral pterygoid processes, the head being the body of the sphenoid bone, and the wings being the greater and lesser wings of the sphenoid. The body of the sphenoid bone is just behind the cribriform plate of the ethmoid bone. The medial anterior surface of the body of the sphenoid bone is flat and is termed the planum (jugum) sphenoidale. The planum sphenoidale is anterior to the sella turcica and connects the two lesser wings of the sphenoid, thus forming a central portion of the anterior cranial fossa. The posterior aspect of the planum sphenoidale is termed the limbus of the planum sphenoidale. Just posterior to the limbus is the chiasmatic groove; then a bony prominence, the tuberculum sellae; and then the sella turcica (Fig. 11-2). The pituitary gland sits in the sella turcica, which (to reiterate) is bounded anteriorly by the chiasmatic groove (the optic chiasm is not located here; however, the lateral portions of the sulcus lead to the optic canals), the tuberculum sellae, and the anterior clinoid processes (part of the lesser wing of the sphenoid), onto which the tentorium cerebelli attaches. The posterior boundary of the sella is the dorsum sellae, from which arise the posterior clinoid processes, onto which the tentorium and petroclinoid ligaments (from the petrous apex) also insert. Behind the dorsum sellae is the clivus, which extends inferiorly to the foramen magnum. Anteriorly, the clivus merges with the sphenoid sinus and the inferior third of the nasopharynx. Its lateral margins are the petro-occipital fissure. Beneath the sella is the sphenoid sinus, which is usually separated asymmetrically by a vertical bony septum. The surgeons figured this out and designed the transsphenoidal hypophysectomy.
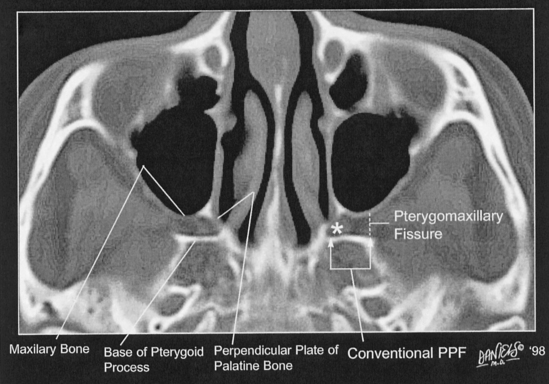
The pterygopalatine fossa is an important conduit for the spread of tumor and infection in and around the skull base. This region can be easily recognized on axial computed tomography (CT) (Fig. 11-3). The pterygopalatine fossa is defined anteriorly by the maxillary bone, anteromedially by the perpendicular plate of the palatine bone, and posteriorly by the base of the pterygoid process. The pterygopalatine fossa is shaped like a deflated balloon, narrower inferior and larger superior. Anteriorly, the pterygopalatine fossa communicates with the orbital apex via the inferior orbital fissure and sphenopalatine foramen (entering the posterosuperior nasal fossa), laterally with the pterygomaxillary fissure (leading to the masticator space), superoposteriorly with the foramen rotundum (and therefore Meckel’s cave and the cavernous sinus), inferoposteriorly with the vidian canal (which communicates with the region of the foramen lacerum), and inferiorly with the greater and lesser palatine canals and foramina (to the palate). An inconstant palatovaginal canal may lead medially to the nasopharynx.
Table 11-1 lists important foramina at the base of the skull and their contents. These need to be learned or relearned.
Table 11-1 Major (and Some Minor) Foramina at the Base of the Skull and their Contents
| Foramen | Contents |
|---|---|
| Superior orbital fissure | Cranial nerves III, IV, first division of V, and VI; orbital branch of middle meningeal artery; sympathetic nerve; recurrent meningeal artery, superior ophthalmic vein |
| Optic canal | Optic nerve, ophthalmic artery |
| Inferior orbital fissure | Infraorbital artery, vein, and nerve (branch of second division of cranial nerve V) |
| Foramen rotundum | Second division of cranial nerve V, artery of foramen rotundum, emissary veins |
| Foramen ovale | Third division of cranial nerve V, lesser petrosal nerve, accessory meningeal artery, emissary veins |
| Foramen spinosum | Middle meningeal artery and vein, recurrent branch of third division of cranial nerve V, lesser superficial petrosal nerve |
| Foramen lacerum | Meningeal branch of ascending pharyngeal artery, nerve of pterygoid canal |
| Foramen of Vesalius | Emissary vein from cavernous sinus to pterygoid plexus |
| Vidian canal | Vidian artery and nerve |
| Jugular foramen | Pars nervosa: cranial nerve IX, inferior petrosal sinus Pars vascularis: Cranial nerves X and XI; jugular bulb |
| Hypoglossal canal | Cranial nerve XII, hypoglossal persistent artery (in rare instance when it is present) |
| Pterygopalatine fossa | Pterygopalatine ganglia (V-2); pterygopalatine plexus |
| Foramen magnum | Medulla oblongata; vertebral artery, anterior spinal artery, posterior spinal artery. |
Let us start from below and work our way up.
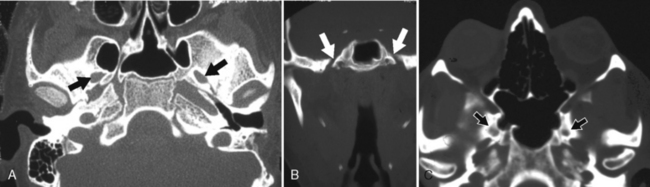
The hypoglossal canal (anterior condyloid foramen) courses obliquely within the occipital bone (Fig. 11-4). Through it runs the hypoglossal nerve and, when present, the hypoglossal artery (a primitive connection between the cervical internal carotid artery at approximately C1–C2 level and the proximal basilar artery). The meningeal branch of the ascending pharyngeal artery as well as a small emissary vein (anterior condyloid) arising from the inferior petrosal sinus may inconstantly also run through this foramen. The jugular tubercles separate the hypoglossal canal from the jugular foramen, with the two regions being about 8 mm apart on the inner surface of the skull. Intracanalicular enhancement on MR is always present, representing multiple emissary venous radicles. Linear filling defects within the enhancement are the hypoglossal nerve rootlets. In addition, dural enhancement can be seen along the margins of the entrance of the canal and anteriorly into the carotid space. Box 11-1 lists the lesions involving the hypoglossal canal.
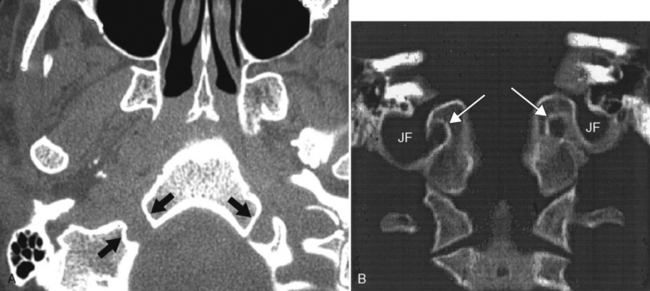
The jugular foramen is demarcated by the petrous portion of the temporal bone anterolaterally and by the occipital bone posteromedially (Fig. 11-5). It is divided into two parts, the pars nervosa (anteromedial) and the pars vascularis (posterolateral), by a bony or fibrous septum (jugular spur). Cranial nerve IX runs lateral to the inferior petrosal sinus within the pars nervosa portion of the jugular foramen. The inferior petrosal sinus runs posterolaterally along the petro-occipital fissure to the pars nervosa and then into the jugular vein (within the pars vascularis). The pars vascularis is the larger of the two compartments and contains cranial nerves X and XI in a common sheath medial to the jugular bulb, which is also in the pars vascularis. (Yes, the pars vascularis has more nerves than the pars nervosa.) The jugular bulb is the confluence between the sigmoid sinus and the jugular vein. It is usually larger on the right side. The petrous portion of the carotid artery is anterolateral to the pars nervosa.
The internal auditory canal is just superior to the jugular foramen. It contains cranial nerves VII and VIII, which are discussed in Chapter 12.
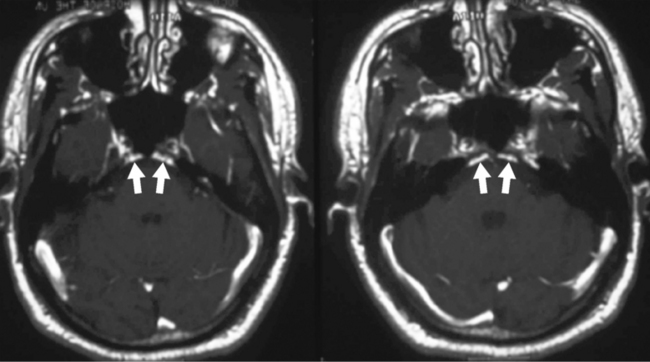
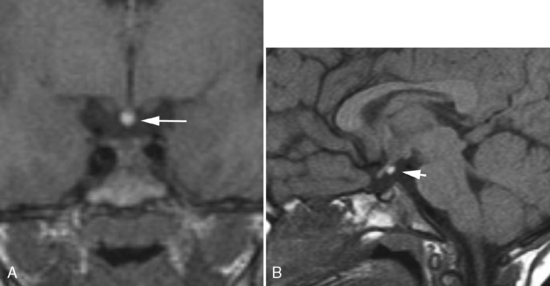
The inferior petrosal sinus can be visualized on contrast CT or magnetic resonance (MR) imaging (Fig. 11-6). The basilar venous plexus connects the superior portions of the inferior petrosal sinuses. Dorello’s canal (petroclival venous confluence) is located just below the petrous apex and is a conduit for cranial nerve VI to reach the cavernous sinus (Fig. 11-7). The canal is located within the inferior petrosal sinus and can be observed on contrast-enhanced axial MR as an unenhanced line crossing the enhanced sinus obliquely. There may be asymmetry and differences in size in this structure. The abducens nerve exits the pontomedullary sulcus, courses through the subarachnoid space, and enters Dorello’s canal and into the cavernous sinus, running just lateral to the intracavernous internal carotid artery. Exiting the cavernous sinus, it enters the orbit through the superior orbital fissure and terminates on the lateral rectus muscle. The dorsal meningeal artery (from the meningohypophyseal trunk), or a branch of it, may also run through Dorello’s canal (see Fig. 11-7). It is located between two dural layers and demarcates an interdural venous confluens. Cranial nerve VI courses in this venous confluens and is separated from blood by a dural or arachnoidal sheath. The posterior portion of the cavernous sinus, the lateral basilar sinus along the clivus, and the superior petrosal sinus fill this region, which then forms the inferior petrosal sinus draining into the jugular bulb.
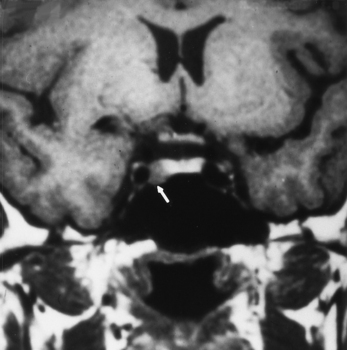
The foramen of Vesalius is an inconstant emissary foramen that can be seen anterior and medial to the foramen ovale. Besides the emissary vein, the ascending intracranial branch of the accessory meningeal artery can enter the middle cranial fossa through the foramen of Vesalius or the foramen ovale (Fig. 11-8).
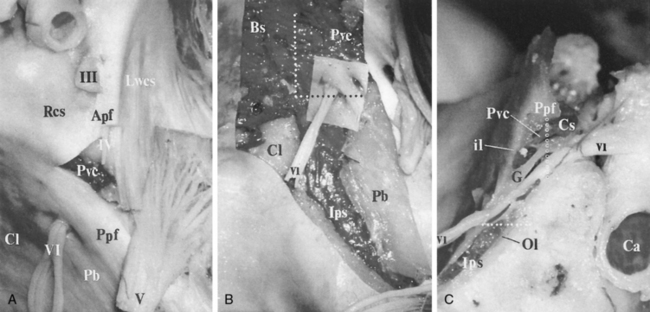
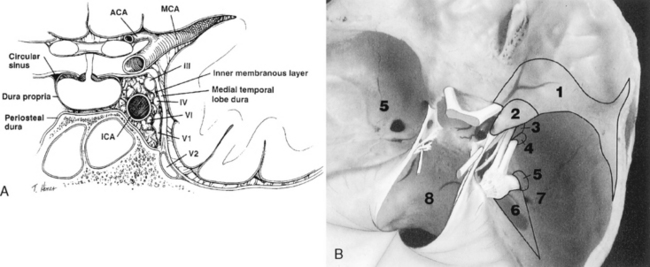
On either side of the sella is the cavernous sinus (discussed later in this section), a trabeculated venous plexus containing cranial nerves III, IV, VI, and the first and second divisions of cranial nerve V. These are located in the lateral portion of the sinus. Cranial nerves III, IV, and the first and second divisions of cranial nerve V are in the lateral wall of the cavernous sinus and maintain that order from superior to inferior in the coronal plane (Fig. 11-9). Cranial nerve VI is medial in the cavernous sinus but lateral to the cavernous carotid artery. Cranial nerve V exits the ventral pons as separate motor and sensory roots at the “root entry zone,” an area often compressed by vascular structures with trigeminal neuralgia. The roots run forward together through the prepontine cistern and exit through the porus trigeminus of the petrous apex. These roots pass over the petrous apex, with the motor root exiting the foramen ovale without merging with the sensory root or gasserian ganglion (semilunar ganglion). The sensory root enters the trigeminal cistern (the space containing cerebrospinal fluid [CSF]), which is in Meckel’s cave, a dural invagination at the posterior aspect of the cavernous sinus. The dural layers of Meckel’s cave demonstrate thin peripheral enhancement. In addition, a discrete semilunar enhancing structure within the inferolateral aspect of Meckel’s cave representing the gasserian ganglion has been observed to enhance, suggesting the lack of a blood-nerve barrier. The gasserian ganglion is a meshwork of sensory neural fibers permeated by CSF from the trigeminal cistern. On CT or MR the CSF in the trigeminal cistern is obviously visualized, and with high-resolution MR the nerve fibers can be seen. The three sensory divisions of the trigeminal nerve leave the gasserian ganglion, with the first and the second divisions running in the lateral wall of the cavernous sinus to exit the superior orbital fissure (along with cranial nerves III, IV, and VI and the superior ophthalmic vein) and foramen rotundum, respectively.
The superior and inferior ophthalmic veins drain into the cavernous sinus via the superior and inferior orbital fissures, respectively; however, there are many variations of this venous drainage pattern. The cavernous sinus is formed by two layers of dura mater. The periosteal layer forms the floor and most of the medial wall, and the meningeal layer (dura propria) forms its roof, lateral wall, and the upper part of its medial wall. The lateral wall may have two layers of dura: a deep layer, which ensheathes cranial nerves III and IV and the first and second divisions of cranial nerve V, and a superficial dural layer. In addition, like most other venous structures in the body, the cavernous sinus has many variations and much controversy about its exact internal venous anatomy. It has been reported that the true cavernous sinus (a large venous channel surrounding the internal carotid artery) exists in only 1% of patients. In the other instances the cavernous sinus is formed by numerous small veins, including (1) the veins of the lateral wall, (2) the veins of the inferolateral group, (3) the medial vein, and (4) the vein of the carotid sulcus (Fig. 11-10).
IMAGING OF THE NORMAL PITUITARY GLAND AND THE PERISELLAR REGION
Intensity is important in MR diagnosis. The anterior lobe of the pituitary gland is isointense to brain on T1WI and T2WI. However, in children younger than age 2 months the pituitary is rounder, larger, and of higher intensity on T1WI than during the rest of infancy. This is most likely related to its high level of metabolic and hormonal function during early infancy, although it has been suggested that the high intensity results from an increase in the bound fraction of water molecules caused by hormone secretion. Hyperintensity on T1WI during pregnancy has also been noted. Reversible hyperintensity has been reported in patients receiving parenteral nutrition (as seen with the basal ganglia secondary to manganese deposition in liver disease). Iron can accumulate in the anterior lobe of the pituitary gland in patients with hemochromatosis and produce low intensity on T2WI and gradient-echo T2*-weighted images (Fig. 11-11).
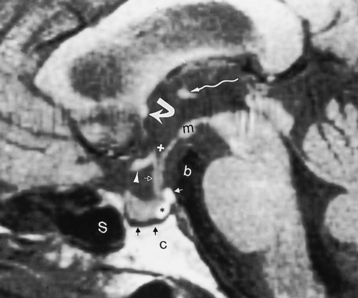
The posterior pituitary gland is high intensity on T1WI and of lower intensity on the T2WI (Fig. 11-12). The precise cause of the high signal in the posterior of the pituitary is unknown but is probably related to the carrier protein (neurophysin) stored in the neurosecretory granules of the posterior pituitary, intracellular lipid in glial cell pituicytes, water interactions with paramagnetic substances, or low molecular weight molecules such as vasopressin or oxytocin. Posterior to the posterior pituitary is a rim of hypointensity, representing cortical bone of the dorsum. Posterior to this hypointense margin is the hyperintensity of fatty marrow in the clivus. High signal intensity has also been observed in the infundibular stalk on fluid-attenuated inversion recovery (FLAIR) images, presumably related to the fluid-rich component (prolonged T2) in the pituitary stalk.
The high intensity of the posterior pituitary gland has been noted to be absent in patients with diabetes insipidus but is identified in only about two thirds of healthy infants. In pituitary dwarfism, a high-intensity nodule on T1WI has been observed at the infundibular apex, possibly from incomplete descent (see below) (Fig. 11-13).
INTRASELLAR LESIONS (BOX 11-2)
Pituitary Adenoma
Pituitary microadenomas (<10 mm) are generally hypointense compared with the normal gland on T1WI and display a variable intensity on T2WI. On CT, the microadenoma is of low density compared with the normal gland with or without enhancement. In about 75% of cases, adenomas in general have associated hormonal abnormality, whereas nonhormonally active lesions become symptomatic because of their size, producing headache, visual disturbances (classically bitemporal hemianopsia), cranial nerve palsy, and CSF rhinorrhea. Usually the diagnosis of pituitary microadenoma may be made without contrast (Fig. 11-14). In most cases, on T1WI the microadenoma appears initially hypointense relative to the normally enhancing pituitary gland (Fig. 11-15). Dynamic MR after contrast has been advocated not only for the detection of microadenomas, but also for imaging what is left of the normal gland in cases of large tumor. The dynamic images obtained within the first minute appear to provide the greatest contrast between enhancing normal gland and pituitary adenoma that does not initially enhance. If a delayed scan (>20 minutes after the injection of contrast) is performed, the tumor may appear hyperintense relative to the gland. However, if extrinsic causes of hyperprolactinemia have been excluded and the patient is considered likely to possess a microadenoma, then an unenhanced MR may be all that is necessary, particularly if the philosophy of the institution is to treat the tumor with drug therapy (bromocriptine). If, however, surgery is contemplated, then contrast is useful to direct the surgical approach to a particular side of the sella.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree